Introduction
Pleural effusions are a frequently encountered problem. The initial step is the distinction between transudates and exudates as this gives a clue of the mechanisms and differential diagnosis 1. Transudates are produced due to disturbance in hydrostatic and oncotic forces and are predominantly seen in congestive heart failure, cirrhosis, nephrotic syndrome and renal failure. Exudates on the other hand result mostly from direct involvement of pleura as seen in malignancy, infections, and connective tissue disorders 2.
Light et al, proposed the criteria for exudates, which are widely accepted and rely on lactic dehydrogenase (LDH) and protein in both serum and pleural fluid 3. Presence of just one of Light’s criteria is enough to make an effusion exudate. Though many new criteria have been developed but none of them is more sensitive or specific than the original Light’s criteria4.
There are no studies reported from Pakistan regarding the use of Light’s criteria for differentiating exudates from transudates. In our setting due to economic restraints and lack of awareness, simultaneous serum LDH and protein levels are not routinely ordered to calculate pleural fluid to serum protein ratio (>0.5 in exudates) and pleural fluid to serum LDH ratio (>0.6 in exudates) as recommended by Light. Mostly pleural fluid protein, LDH and cell count are measured to differentiate between transudates and exudates.
We conducted this retrospective analysis to investigate the utility of conventional criteria in identifying true exudates and transudates. We also separated out one of the Light’s criteria for exudates i.e. isolated pleural fluid LDH level more than 200 IU/L and compared with fluid protein level, total white blood cell (WBC) count and red blood cell (RBC) count in the pleural fluid to find a cost effective but more state of the art way of categorizing pleural effusions.
Materials and Methods
We conducted a retrospective analysis of all the patients who underwent diagnostic thoracentesis for the period of one year. The laboratory and clinical information was gathered from the medical records. The cause of pleural effusions was identified using following predetermined criteria.
Congestive heart failure (CHF): CHF was diagnosed when there was cardiomegaly on chest X-ray, pulmonary venous congestion on radiology, peripheral edema and response to optimal treatment and absence of malignancy, pulmonary infiltrates, sputum and pleuritic chest pain.
Liver Cirrhosis: When clinical and laboratory findings were suggestive of liver disease and other causes of pleural effusion were ruled out.
Malignant effusions: were diagnosed either by positive fluid cytology or pleural biopsy. Effusions in which atypical cells were identified with evidence of malignancy elsewhere were also considered malignant provided there was no other reason for pleural effusion.
Tuberculous pleuritis: was diagnosed by identifying granulomas on pleural biopsy or response to empiric anti-tuberculous treatment.
Para-pneumonic/ Empyema: was diagnosed when there was a febrile illness with purulent sputum, pulmonary infiltrates and response to treatment, or identification of organism in effusion; empyema, associated with frank pus in pleural cavity.
Others:
Transudates vs Exudates: We used the LDH criteria of more than 200 IU/L, as proposed by Light et al, as exudate. We also used conventional criteria including pleural fluid WBC count more than 1000 per mm3; protein level more than 3 gram/dL and RBC count more than 10,000 per mm3.
Laboratory Measurements:
LDH: LDH was measured using automated chemistry analyzer (cobas-mira). LDH catalyses the conversion of pyruvate to lactate. NADH is oxidized to NAD in the process. The rate of decrease in NADH is directly proportional to LDH activity and it is determined photometrically.
Pleural Fluid Protein: Total protein was measured by automated clinical analyzer (Cobas-mira). Biuret method was used to measure the protein level, which is a colorimetric technique specific for proteins.
Cell count: Both WBC and RBC were estimated by counting the cells in improved Neubauer ruled chamber.
Statistical Methods:
For each of the four criteria mentioned above for identifying exudate we calculated following statistical measures: sensitivity = TP/(TP+FN); specificity = TN/(TN+FP) ; positive predicted value = TP/(TP+FP); negative predictive value = TN/(TN+ FN); and accuracy = (TP+TN)/(TP+TN+ FP+FN) where TP is the number for true positive diagnosis, TN the number of true negative diagnosis, FP the number of false positive diagnosis and FN the number for false negative diagnosis. We used these statistics with reference to exudates. Quantitative data are represented as mean±1SD and mean±1SE.
Results
Sixty-two patients who underwent thoracentesis were reviewed during one-year period and out of these 16 were excluded from final analysis, as they did not meet the pre-determined clinical criteria mentioned before. The causes for 46 patients with pleural effusion are shown in table-1. Malignancy, pneumonia, empyema and tuberculosis were the most common diagnosis observed. There were 25 men and 21 women with mean age of 49.3 years (SD±19.1). Eight patients had transudate with five men and three women. Thirty-eight patients had an exudate (twenty men and eighteen women) with mean age of 45.6 years (SD±18.7).
Table 1: Distribution of various diagnosis causing pleural effusions
|
Etiology |
No of patients |
Percentage |
|
Transudates CHF Cirrhosis Exudates Malignancy Parapneumonic Empyema Tuberculosis Others |
6 2
13 9 5 8 3 |
13 4.3
28.3 19.6 10.8 17.4 6.5 |
Table-2 illustrates sensitivity, specificity, positive predictive value, negative predictive value and accuracy of different parameters analyzed in the diagnosis of exudates. LDH was found to be the most sensitive (97.2%) of all the criteria tested. The specificity for LDH and protein criteria were 89% which was less than WBC count (100%). We used LDH more than 200 IU/L as a representative of classic Light’s criteria. As shown the classic Light’s criteria of LDH showed the best accuracy (95%), followed by pleural fluid protein (79%), WBC count (60.5%), and RBC count (41.8%).
Table 2:Utility of different parameters in diagnosing exudative effusions
|
Parameter TP FP TN FN n Sensitivity Specificity PPV NPV Accuracy % % % % % |
|
LDH>200 36 1 8 1 46 97.2 89 97.2 89 95.6 Protein>3 27 1 8 10 46 72.9 89 96.4 44.4 76.0 WBC>1000 18 0 9 19 46 48.6 100 100 32.1 58.7 RBC>10,000 19 2 7 18 46 51.3 77.7 90.4 28 56.5 _______________________________________________________________________________ TP=true positive, TN=true negative, FP= false positive, FN= false negative, PPV= positive predictive value, NPV=negative predictive value |
Applying conventional criteria of pleural fluid protein, WBC and RBC count the percentage of falsely classified exudates and transudates are shown in figure-1 which also depicts falsely classified effusions by applying Light’s criteria of pleural fluid LDH. As the figure shows the percentage of falsely classified transudates was much higher with pleural fluid protein, RBC count and WBC count as compared with LDH.
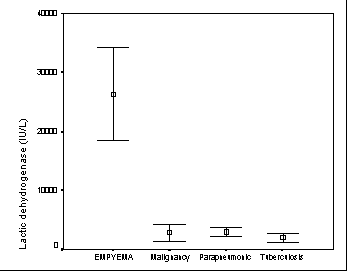
Figure-2 shows mean LDH level and standard error (mean±SE) in most commonly found diagnosis. As expected, LDH levels were significantly higher in empyema and parapneumonic effusions as compared with other causes of exudates
Figure 1: Percentage of exudates and transudates misclassified using various criteria
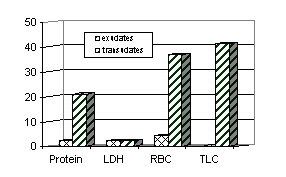
Figure 2: Lactic Dehydrogenase level in different pathologic conditions (Mean±SE)
Discussion
Characterization of pleural fluid as exudate or transudate is usually the first step in the evaluation of pleural effusion. Once transudate is diagnosed no further workup is generally recommended5. But exudative effusions merit further probing to find the cause. Transudates generally are a result of increased hydrostatic pressure (congestive heart failure), decreased oncotic pressure (nephrotic syndrome) or lymphatic obstruction (malignancy). Exudates are due to processes, which directly involve pleura (e.g. parapneumonic effusions, malignancy, pulmonary infarction etc)1,5,6.
In this study, pleural fluid LDH was the most accurate parameter to differentiate exudate from transudate. Light’s criteria include pleural fluid LDH to serum LDH ratio (>0.6 in exudates), pleural fluid protein to serum protein ratio (>0.5 in exudates) and isolated pleural fluid level more than 200 IU.3 Presence of one of these criteria is sufficed to make a pleural effusion as an exudate. As most of the times simultaneous serum LDH and serum protein concentrations are not ordered at our institution, we opted to use isolated pleural fluid LDH as the representative of Light’s criteria. When compared with conventional parameters i.e. pleural fluid protein more than 3 gram/dl, TLC more than 1000 cubic millimeter and RBC count more than 10,000, Light’s classic criteria of isolated LDH proved to be the most accurate.
In a recent study, isolated pleural fluid LDH was found to be more accurate than LDH and protein ratio in identifying exudates or transudates7. Pleural fluid protein depends on serum protein concentration (thus the use of pleural fluid to serum protein ratio). Lack of reliability of protein ratio has been shown before for separation of pleural effusions8. On the other hand, pleural fluid LDH does not depend on serum LDH concentration7. Pleural fluid LDH can come from active or dead mesothelial cells and also from inflammatory cells involving pleura (as in malignancy, infarction and inflammations). So increasing pleural LDH is a sensitive marker for exudative process and there is no need to use LDH ratio in diagnostic separation of pleural effusion 9,10.
Pleural fluid protein level of more than 3 gm/dl though still used extensively by clinicians especially in our country, is not a reliable parameter as shown in our study as well and should not be used alone to categorize pleural effusion. Pleural fluid protein to serum protein ratio is more reliable but it not only adds to cost and but also has many limitations that merits its use. Though many other criteria have been developed including pleural fluid cholesterol11, pleural fluid albumin gradient12 and pleural fluid bilirubin to serum bilirubin ratio13, their sensitivities and specificities are not better than classic Light’s criteria. These new criteria may be helpful in special circumstances where use of diuretics in congestive heart failure can falsely change LDH and protein ratios in favor of exudate.
In conclusion, pleural fluid LDH is the most accurate test in the diagnostic separation of transudates and exudates. As pleural fluid LDH does not depend on serum LDH, there is no need to use LDH ratio in identification of pleural effusions. Moreover, ordering pleural fluid protein is also not justified in the light of this and other studies in literature. Following the strategy of relying on pleural fluid LDH alone will also result in significant cost savings and is highly recommended.
References:
1. Bartter T, Santarelli R, Akers SM, Pratter MR. The evaluation of pleural effusion. Chest 1994; 106:1209-1214
2. Light RW. Pleural diseases. 3rd ed. Baltimore: William & Wilkins. 1995, 7-17
3. Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusion: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507-513
4. Vives M, Porcel JM, Vincente de Vera M, Ribelles E, Rubio M. A study of Light’s Criteria and possible modifications for distinguishing exudative from transudative pleural effusions. Chest 1996;109:1503-1507.
5. Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions: experience with clinical studies, 1986 to 1990. Chest 1995;107:1598-1603
6. Burgess LJ, Maritz FJ, Taljaard LL. Comparative analysis of the biochemichal parameters used to distinguish between pleural transudates and exudates. Chest 1995; 107:1604-1609.
7. Joseph J, Badrinath P, Basran GS, Saha SA. Is the pleural fluid transudate or exudates? A revisit of the diagnostic criteria. Thorax 2001;56:867-870.
8. Chakko SC, Caldwell SH, Sforza PP. Treatment of congestive heart failure. Its effect on pleural fluid chemistry. Chest 1989;95:798-802.
9. Whitaker D, Papadimitriou JM, Walters MN. The mesothelium. A histochemical study of resting mesothelial cells. J pathol 1980;132:273-284
10. Vergnon JM, Guidollet J, Gateau O, Ripoll JP, Collet P, Lousit P, et al. Lactic Dehydrogenase isoenzyme electrophoretic patterns in the diagnosis of pleural effusion. Cancer 1984;54:507-511.
11. Hamm H, Brohan U, Bohmer R, Mismahl HP. Cholestrol in pleural effusions: a diagnostic aid. Chest 1987;92:296-302.
12. Roth BJ, O’Meara TF, Cragun WH. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest 1990;98:546-549.
13. Meisel S, Shamiss A, Thaler M. Pleural fluid to serum bilirubin concentration ratio for the separation of transudates from exudates. Chest 1990;98:141-144.