INTRODUCTION
Use of tobacco is a world wide
problem and the traditional methods of tobacco use are smoking, snuffing,
chewing and dipping. Chronic use of tobacco is causally linked to a variety of
serious diseases, ranging from coronary artery disease to lung cancer.1
However it was in 18th century when it was
discovered that smoking increases the activity of salivary glands. Indeed, this
observation has been made by every one who begins smoking. It has also been
observed that some tolerance develops to the salivary effects of smoking
because habitual smokers do not salivate as do novice smokers in response to
smoking.2 However, it has also been seen that there is no difference
in the secretion rate of saliva between smokers and non-smokers.3 It
was observed that regular, but not immediate, smoking did not cause any
significant change in the salivary flow rate.4
Mostly
it is believed that long term use of tobacco depresses or inactivates the taste
receptors and salivary reflex. Presumably, this might lead to altered taste
receptors response and hence to changes in salivary secretion. The present
study was designed to document these changes, if any.
MATERIAL AND METHODS
The subjects were selected from
the students of Basic Medical Sciences Institute (BMSI),
Jinnah Post Graduate Medical Centre (JPMC) and the
general population of
The subjects
were divided into smokers, pan (tobacco-betel-lime quid) chewers, niswar (moist oral snuff) dippers and non-tobacco users as
controls. Each group comprised of 20 apparently healthy male adults. All the
subjects were well matched with respective to age (25-30 years) and the
duration of beginning tobacco use (5-7 years). Subjects in the habit of more
than one type of tobacco use or bad orodental hygiene
or with too little salivary secretion were not included in the study.
Before sampling,
each subject was briefed about the procedure and instructed to wash his mouth
and gargle with plain water. The saliva of each subject was collected (for 10
minutes) under resting condition and following application of crude nicotine
solution (50 ml
of 1% v/v) and citric acid solution (50 ml of 1% w/v) to the tip of
his tongue. Crude nicotine was extracted from tobacco5 and citric
acid was obtained from the Physiology Department of BMSI,
JPMC,
RESULTS
Taste sensations
The appreciation of taste
sensation was different in case of nicotine stimulation from that in case of
citric stimulation. Each of the subjects of all groups perceived a bitter
burning taste when nicotine solution was applied to the tip of his tongue.
However, the application of citric acid caused a sour burning taste in all the
subjects of all groups.
Salivary flow rate
The mean salivary flow rate of
controls (0.44 + 0.04ml/min) smokers (0.49 + 0.05 ml/min) pan
chewers (0.47 + 0.04 ml/min) and niswar
dippers (0.47 + 0.04 ml/min) did not show great variation from one
another and no statistically significant difference (P>0.05) was observed
when the chronic tobacco user groups were compared with controls under resting
condition (Figure 1).
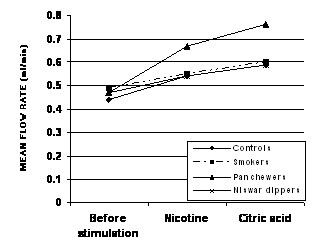
Figure-1: Change in mean
salivary flow rate of controls, smokers, pan chewers & niswar
dippers, following stimulation with 50µl of 1%(v/v) crude nicotine & 50µl
of 1%(w.v) citric acid
Following stimulation
with nicotine, there was a gradual increase in the flow of saliva of all groups
which then gradually declined to the resting level. The mean flow rates were
0.54 + 0.04 ml/min in control, 0.55 + 0.05 ml/min in smokers,
0.67 + 0.04 ml/min in pan chewers and 0.54 + 0.04 ml/min in niswar dippers. The increase in the mean flow rate of
controls (22.73%) smokers (12.25%) pan chewers (42.55%) and niswar
dippers (14.89%) was statistically significant (P<0.05) in controls, highly
significant (P<0.005) in pan chewers but not significant (P>0.05) in the
other two groups. When the mean salivary flow rates of the chronic tobacco
users were compared with the
corresponding mean salivary flow rates of controls, a statistically significant
difference (P<0.05) was observed in case of pan chewers only (Table 1).
After stimulation with citric acid, an
abrupt rise was seen in the flow of saliva of all groups and than gradually
declined to the basal level. The mean flow rates were 0.59 + 0.05 ml/min
in controls, 0.60 + 0.05 ml/min in smokers, 0.76 + 0.04 ml/min in
pan chewers and 0.59 + 0.04 in niswar dippers.
The increase in the mean flow rates of controls (34.09%), smokers (22.45%), pan
chewers (61.70%) and niswar dippers (25.53%) was not
statistically significant (P>0.05) in controls and niswar
dippers and highly significant (P<0.005) in pan chewers. On comparison the
mean salivary flow rates of chronic tobacco user groups with that of controls,
the only statistically significant difference (P<0.5) was observed in case
of pan chewers (Table 1).
Table-1. Comparison
of mean salivary flow rate (ml/min) of controls, smokers, pan chewers and niswar dippers, before and after stimulation with 50µL of
1% (v/v) Crude Nicotine and 50µL of 1% (w/v) Citric acid
|
Group |
Mean salivary flow rate (ml/min) ±S.E. |
||
|
Before stimulation |
Following stimulation with |
||
|
Nicotine |
Citric
acid |
||
|
Controls |
0.44
± 0.04 |
0.45
± 0.04 (22.73%)# |
0.59
± 0.05 (34.09%)# |
|
Smokers |
0.49
± 0.05 |
0.55
± 0.05 (12.25%) |
0.60
± 0.05 (22.45%) |
|
Pan
chewers |
0.47
± 0.04 |
0.67
± 0.04* (42.55%)## |
0.76
± 0.04* (61.70%)## |
|
Niswar
dippers |
0.47
± 0.04 |
0.45
± 0.04 (14.89%) |
0.59
± 0.05 (25.53%)# |
Percent (%) increase is given in parenthesis
*p< 0.05 as compared with
corresponding mean value in controls.
# p<0.05 and ##p<0.005 as compared with its mean value
before stimulation
DISCUSSION
The
salivary secretion is a complex process and its flow and composition vary
greatly under different conditions. On the basis of some experiment it was
thought that saliva collected routinely in the laboratory as “resting” saliva
is in fact stimulated or activated secretion and gross variation in the rate of
its secretion are due to fluctuation in intensity and frequency of internal
stimulation.8 It is said that the secretion of saliva from the
salivary glands is generally elicited only in response to stimulation of the
automatic innervation to the glands or in response to
drugs that mimic the actions of automatic innervation.9 It was also
found that different chemicals stimulate the salivary secretion differently.10 It has been observed
that the pattern of taste sensations and salivary secretion in man following application
of compounds, like nicotine and citric acid, to the tongue, which activate
lingual sensory neurons, differ not only between the agents used but also
between different sites of application.11 It is suggested that oral
mucosal wetness and minor salivary gland secretion could be influenced by
various factor differently according to mucosal sites.12 Moreover,
temperature of stimulating substances also affects salivary secretion because
the stimuli in the form of ice were the most effective and liquids at 370C were least
effective in stimulating salivary flow.13
The present work revealed that the appreciation of taste sensations evoked by the application of
nicotine and citric acid to the tongue were different in the same
individual but no subjective difference was observed between tobacco users and
non-tobacco users. In this regard the taste receptors response to either of the
compound was similar in all groups.
It
was noted that buffering response in smokers in response to drinking acidic
carbonated beverages is 20% lower than in non-smokers. On this basis an in
activation of the taste receptors by nicotine was suggested as an explanation
for this depression of the salivary reflex.14 Although
we have not studied the buffering response of saliva in our study, yet we were
unable to find any significant difference in the salivary response to
stimulating substances between tobacco users and non-tobacco users. It seems,
therefore, somewhat unreasonable to suggest an inactivation of the taste
receptors merely on the basis of lowered buffering response of saliva in
smokers. Moreover it was also found that the pH of stimulated whole saliva, in
both sexes, was lower in smokers than non-smokers4. In our opinion,
this lowered buffering response to acidic carbonated beverages might be due to
this acidic pH in these individuals. Similarly no statistically significant
different was observed for either over all taste sensitivity or for the
specific taste primaries between smokers and non-smokers.15
We
found that lingual apex application of nicotine and citric acid was associated
with a rise in salivary secretion rate but the salivation response to citric
acid was abrupt and more pronounced as compared to nicotine proving that citric
acid is more potent and quicker in its action. However, the flow rates in pan
chewers were comparatively higher and significant. The authors of one study,
who also found similar higher flow rates in pan chewers, were of the view that
this might actually be due to increased salivary gland mass induced as a result
of chronic chewing or due to chronic exposure to one or all of the constituents
of tobacco-betel-lime quid.16 However, it seems reasonable to us
that tobacco on its part, might not be responsible for the increased gland mass
induction in pan chewers, for it would also bring similar changes in smokers
and niswar dippers.
The
effect of nicotine on the taste nerve apparatus appears to be initial
stimulation followed by depression2. In the study under
consideration the initial increase in the flow of saliva following stimulation
by both nicotine and citric acid and then gradual decline also gives similar
impression but before establishing such an opinion it must be bore in mind that
increased flow of saliva also gradually washes the stimulating substances.
We
have seen, that the behavior of taste sensations and
salivary secretion in chronic tobacco users are not much different from that in
non-tobacco users. In view of the previous studies and our own experimental
work, it is therefore concluded that long term use of tobacco does not
adversely affect the taste receptors, salivary reflex and salivary secretion.
REFERENCES
1.
Jaffe JH. Drug
Addiction and Drug Abuse, In: Gilman AG, Goodman LS, Ral,
TW, Murad F (Eds). The Pharmacological Basis of Therapeutics.
2.
Larson PS, Haag HB, Silvette H. Tobacco,
Experimental and Clinical Studies.
3.
Heintz U. Secretion rate, buffer effect and number of
lactobacilli and streptococcus mutans of whole saliva
of cigarette smokers and non-smokers.
Scand J Dent Res 1984; 1: 294-301.
4.
Parvinen T. Stimulated Salivary flow rate, pH and lactobacillus
and yeast concentrations in non-smokers and smokers. Scand J Dent Res 1984; 92: 315-8.
5.
6.
Chaudary SM. Introduction to Statistical Theory (Part 2). Ilmi Kitab Khana.
7.
Ganong WF. Review of Medical Physiology. Medical
Books/McGraw-Hill Medical Publishing Division. 2001: 714-6.
8.
Schneyer LH, Pigman W, Hanana L, Gilmore RW. Rate of flow of human parotid,
sublingual and sub-maxillary secretions during sleep. J Dent Res 1956;35:109-14.
9.
Schneyer LH, Young JA, Schneyer CA.
Salivary secretion of electrolytes. Physiol Rev 1972;
52: 720-77.
10.
Jones CJ, Wilson
SM. The effect of automatic agonists and nerve stimulation on protein secretion
from the rat submandibular gland. J Physiol 1985;385:65-73.
11.
Engstom MD, Fredholman BB, Larsson O,
Lundberg JM, Saria A.
Autonomic Mechanisms underlying capsaicin induced oral sensations and
salivation in man. J Physiol 1986;373:87-96.
12.
Won S, Kho H, Kim
Y, Chung S, Lee S. The effect of age on taste sensitivity. Arch Oral Biol 2001;46:619-24.
13.
Dawes C, O’Connor
AM, Aspen JM. The effect on human salivary flow rate of the temperature of a
gustatory stimulus. 2000; 45: 957-61.
14.
Oster RH, Prouth LM, Shipley ER, Pallack BR, Bradley JE. Human buffering rate measured in
situ in response to an acid stimulus found in some common beverages. J Appl Physil. 1953-1954; 6:
348-54.
15.
Cooper RM, Bilash I, Zubek JP. The effect of
age on taste sensitivity. J Gerontol 1959;
16.
Reddy MS, Naik SR, Bagga OP, Chuttani HK. Effect of chronic tobacco-betel-lime “quid”
chewing on human salivary secretions. Am J Clin Nutr 1980;33:77-80.