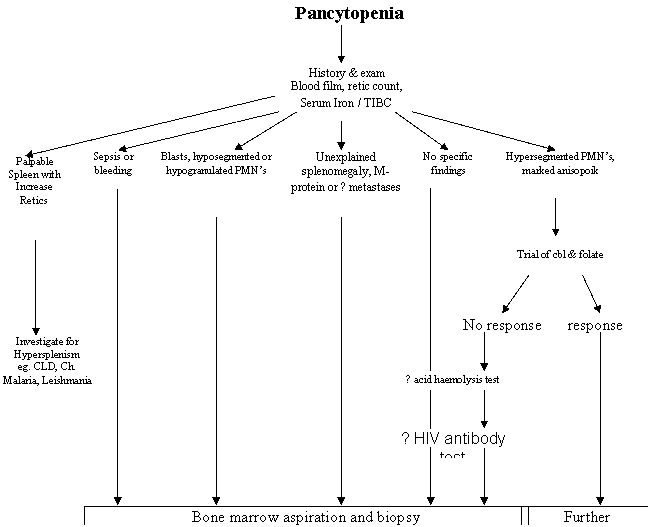
INTRODUCTION
Pancytopenia means a disorder in which all 3 blood elements (red blood
cells, white blood cells and platelets) are decreased than normal.[1] Although it is a common clinical problem with an extensive differential
diagnosis, there is a relatively little discussion of this abnormality in major
textbooks of internal medicine and hematology.[2],[3] Pancytopenia can be due to decrease in hemaopoietic cell production in
the bone marrow e.g. by infections, toxins, malignant cell infiltration or
suppression or can have normocellular or even hypercellular marrow, without any
abnormal cells, e.g. ineffective hematopoiesis and dysplasia, maturation arrest
of all cell lines and peripheral sequestration of blood cells.[4]
Few clear recommendations can be found as to the
optimal investigative approach to pancytopenia. Some experts suggest that
marrow examination is essential to the diagnosis, but it has not been
established whether the procedure is necessary in all pancytopenic patients.1,2
Common questions that a healthcare professional asks
are 1) What are the most common causes of pancytopenia? and 2) What is the best
diagnostic approach to the pancytopenic patient? In the present study, we have
attempted to answer these questions by doing investigation of pancytopenic
patients in a general medical ward. Our study was limited by its restriction to
hospitalized patients. On the basis of our findings and available literature,
we have formulated a diagnostic approach to the problem of pancytopenia.
MATERIAL AND METHODS
Pancytopenia was diagnosed in the presence of anemia
(hematocrit value <0.35 in women, <0.40 in men), leucopenia (WBC Ł 3.5 x 109/L) and thrombocytopenia (platelets < 150 x 109/L).
This
study was carried in Medical Unit II of Holy Family Hospital, Rawalpindi from
October 2001 to October 2002. All admitted patients with pancytopenia were
studied and a total of 100 adult patients were selected for the study by
non-probability convenient sampling. In all patients, a detailed relevant
history including the treatment history, history of drug intake, radiation
exposure, along with a physical examination of pallor, jaundice, hepatomegaly,
splenomegaly and lymphadenopathy, was taken.
Patients on cancer chemotherapy were excluded.
Blood
counts obtained prior to transfusion were done on an automated blood analyser
(Sysmex). In cases of very low counts, manual methods were used which included
total leucocyte count, red cell and platelet count, using improved Neubauer
chamber. Differential leucocyte count and red cell morphology was done manually
by staining the blood smears by May-Granwald-Giemsa stains. Bone marrow
aspiration and wherever required, a trephine biopsy were also performed. They
were independently reviewed by one investigator without knowledge of the
patient’s clinical presentation. Anisocytosis and poikilocytosis were graded
according to the degree of variation in size and shape.
Findings
of aspiration and trephine biopsies were interpreted in the light of history,
clinical examination and peripheral blood findings. Standard morphologic
criteria were used in diagnosis ([5],[6]). Bone marrow examination was done in 70
patients and 30 patients in whom bone marrow examination was not done were
either very ill or were meeting other criteria of diagnosis or expired before
the procedure.
Statistical methods
include descriptive statistics (mean, median, SD). Cross tabulation was carried
out to find out the correlation among different variables. Analysis was done on
SPSS v10 for windows.
RESULTS
The age of the patients ranged from 12 to 82 years with a mean age of
36.7 years, in which 53% were males. The
aetiological breakup of all patients is given in Table 1. Two patients were the
suspected cases of viral hemorrhagic fever (VHF) among which 1 patient was
diagnosed as having Cremean Congo hemorrhagic fever. Thirteen (13%) patients
expired, among which 8 patients had septicemia and disseminated intravascular
coagulation (DIC), 2 with VHF, 2 with disseminated malginancy and one with
acute leukemia. Two patients had a drug history of interferon and ribavarin.
Clinical features of patients with pancytopenia are
listed in Table 2. Patients with megaloblastic anemia had a mean age of 38.6
years and belong to almost all age groups. Similar ages (mean 35.6 years) are
seen in Aplastic Anemia as well. Patients with hypersplenism had a slightly
higher mean age of 41.37 years.
WBC counts did not vary between different diagnostic
categories. However, absolute neutrophil count (ANC) less than 500 /µl was seen
in 14 (14%) patients, among which 6 (15.3%) had megaloblastic anemia, 3 (37.5%)
had aplastic anemia, 2 (40%) had myelodysplasia and 1 (50%) with leukemia.
Platelet counts ≤ 10 x 109/L
were noted in 37.5% (n=3) of patients with aplastic anemia verus 7.6% (n=3) of
those with megaloblastic anemia. Of 11 patients with platelet counts ≤ 10
x 109/L, 6 (54.5%) presented with bleeding; and 2 of these
8 had aplastic anemia and 1 patient with megaloblastic anemia. Anemia was most
marked in patients with aplastic anemia,
acute leukemia and septicemia. (Figure 1). There was no marked variation
seen in the ESR among various diseases.
The MCV was ≥ 100fL in 41 patients (41%).
Highest MCV noted was 135fL seen in megaloblastic anemia. MCV values > 100fL
and > 110fL were more frequent in patients with megaloblastic anemia than in
those with aplastic anemia or hypersplenism (Figure 2). The MCV was < 100fL
in 6 (15.3%) patients with megaloblastic anemia (Figure 2). The lowest MCV was
58fL seen in iron deficiency anemia.
Moderate to marked anisopoikilocytosis, microcytosis
and fragmented RBCs were detected in all of the pancytopenic disorders, but
were most prominent in megaloblastic anemia (data not shown). Macrocytosis was
noted in 35 (89.7%) patients with megaloblastic anemia and 12 (63.1%) with
hypersplenism, 4 (50%) with aplastic anemia and 4 (80%) with myelodysplasia.
Microcytosis was seen in 3 (7.6%) patients with megaloblastic anemia, 7 (36.8%)
with hypersplenism, 1 (12.5%) with aplastic anemia and 1 (20%) in
myelodysplasia.
Hypersegmented neutrophils were noted in the blood films of 36 (92.3%) patients of 39 patients with megaloblastic anemia.
To calculate the sensitivity, specificity and
predictive values of blood findings for megaloblastic anemia, data were
examined for 100 pancytopenic patients whose diagnosis was established by
marrow examination or response to cobalamin and folate therapy (Table 3).
Neutrophil hypersegmentation had high specificity and predictive value for
megaloblastic anemia. In contrast, MCV > 110fL was specific but not
sensitive.
DISCUSSION
Pancytopenia is not an uncommon hematological problem encountered in our
clinical practice and should be suspected on clinical grounds when a patient
presents with unexplained anemia, prolonged fever and tendency to bleed. In all
cases, megaloblastic anemia constituted the largest group, and also seen in
conjunction with hemolytic anemia and septicemia. Hypersplenism secondary to portal
hypertension (cirrhosis) was the second most common diagnosis. Aplastic anemia,
septicemia and myelodysplasia were other common causes of pancytopenia (Table
1).
The approximately comparable series of pancytopenia is
from Savage et al2,
Iqbal et al4
and Qazi et al[7]. In all these studies, megaloblastic was found to be the largest cause
of pancytopenia. In contrast to local studies, significant contributor of
pancytopenia is HIV infections2,
compared to only 1 patient in our study. Study done by Iqbal et al also
included pediatric population and reported leishmaniasis in 4.8% of cases as
compared to none seen in our study.4
The major cause of megaloblastic anemia in our study is fever as 56.4% and chronic diarrhea (Table 2) as 38.5%. The percentage of chronic diarrhea in our study is not that common as in other studies.4,7
Table 1: Etiology of
Pancytopenia
|
|
Male |
Female |
Total |
|
Megaloblastic Anemia |
|
|
|
|
16 |
11 |
27 (27%) |
|
|
with Hemolytic
Anemia |
|
|
|
|
4 |
3 |
7 (7%) |
|
|
with Sepsis |
|
|
|
|
4 |
1 |
5 (5%) |
|
|
Total |
24 |
15 |
39 (39%) |
|
Hypersplenism |
|
|
|
|
With Cirrhosis |
|
|
|
|
6 |
6 |
12 (12%) |
|
|
With Chronic Malaria |
1 |
2 |
3 (3%) |
|
Undiagnosed |
1 |
3 |
4 (4%) |
|
Total |
8 |
11 |
19 (19%) |
|
Aplastic Anemia |
6 |
2 |
8 (8%) |
|
Myelodysplasia |
|
|
|
|
3 |
2 |
5 (5%) |
|
|
Septicemia and DIC |
|
|
|
|
3 |
2 |
5 (5%) |
|
|
Iron Deficiency Anemia with Leucopenia |
|
|
|
|
1 |
4 |
5 (5%) |
|
|
Connective Tissue Disease (SLE) |
|
|
|
|
- |
3 |
3 (3%) |
|
|
Disseminated Tuberculosis |
|
|
|
|
1 |
2 |
3 (3%) |
|
|
AIDS and Septicemia |
|
|
|
|
- |
1 |
1 (1%) |
|
|
Disseminated Malignancy |
|
|
|
|
2 |
- |
2 (2%) |
|
|
Hypoplastic Marrow |
|
|
|
|
1 |
1 |
2 (2%) |
|
|
Viral Hemorrhagic Fever (CCHF) |
|
|
|
|
- |
1 |
1 (1%) |
|
|
Acute Lymphocytic Leukemia |
|
|
|
|
1 |
- |
1 (1%) |
|
|
Mixed Leukemia |
|
|
|
|
- |
1 |
1 (1%) |
|
|
Undiagnosed |
|
|
|
|
3 |
2 |
5 (5%) |
|
|
Total |
|
|
|
|
53 |
47 |
100 |
|
Table 2.: Clinical Features of Patients presenting with Pancytopenia
(Percentages are calculated from totals of each disease)
|
Clinical Features |
Megaloblastic Anemia * |
Hypersplenism |
Aplastic Anemia |
Others ‡ |
|
Pallor |
100% |
100% |
100% |
100% |
|
Fever |
22
(56.4%) |
11
(57.4%) |
4
(50%) |
22 |
|
Diarrhea |
15
(38.5%) |
1
(5.3%) |
- |
6 |
|
Anorexia |
14
(35.9%) |
1
(5.3%) |
- |
4 |
|
Jaundice |
7
(17.9%) |
7
(36.8%) |
- |
2 |
|
Lymphadenopathy |
7
(17.9%) |
- |
1
(12.5%) |
8 |
|
Bleeding † |
12
(38.8%) |
8
(42.1%) |
4
(50%) |
9 |
|
Splenomegaly |
6
(15.4%) |
19
(100%) |
- |
9 |
|
Hepatomegaly |
7
(17.9%) |
4
(21.1%) |
- |
13 |
|
Dyspnea |
4
(10.3%) |
1
(5.3%) |
4
(50%) |
5 |
|
Weight Loss |
6
(15.4%) |
1
(5.3%) |
1
(12.5%) |
6 |
* Other causes with secondary megaloblastic anemia also included
† Upper GI Bleeding, Epistaxsis, Petechie,
Bleeding Gums are included
‡ Percentages not calculated because of different totals
Table 3: Sensitivity, Specificity
and Predictive Values for various laboratory findings in Diagnosis of
Megaloblastic Anemia in 100 pancytopenic patients *
|
Lab finding |
Sensitivity (%) |
Specificity (%) |
Positive Predictive Value (%) |
Negative Predictive Value (%) |
|
MCV > 100 fL |
82 |
91.8 |
86.4 |
88.8 |
|
MCV > 110 fL |
51.2 |
100 |
100 |
76.2 |
|
Neutrophil Hypersegmentation |
92.3 |
98.3 |
97.2 |
95.2 |
* Megaloblastic anemia
excluded in 61 patients by marrow examination or response to therapy
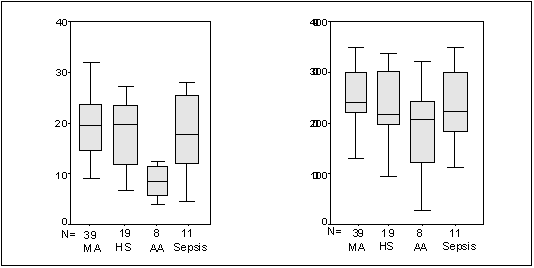
Figure 1: Box plots of
Hematocrit and Total leucocyte counts
(MA = Megaloblastic Anemia, HS
= Hypersplenism, AA = Aplastic Anemia)
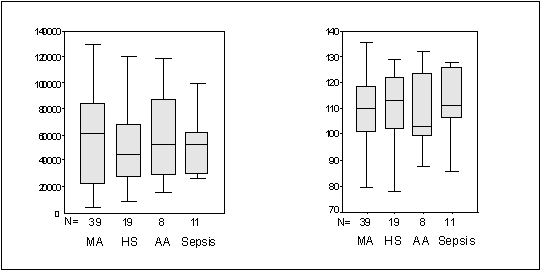
Figure 2. Box plots of
Platelets and MCV
(MA = Megaloblastic Anemia, HS
= Hypersplenism, AA = Aplastic Anemia)