J Ayub Med Coll Abbottabad 2004;16(2)
Close Association between parathyroid hormone and left ventricular function and structure in end-stage renal failure patients under maintenance hemodialysis
Hamid Nasri, Azar Baradaran
Shahrekord University of Medical Sciences, Shahrekord ,Iran. *Department of Biochemistry , The Center of Research and Reference Laboratory of Iran, Hospital Bou-Ali, Tehran. Iran
Background: Cardiovascular risk factors are a significant burden in end-stage renal disease patients under hemodialysis and are the leading cause of death among these patients. The influence of parathyroid hormone (PTH) on myocardial function as a toxin of uremia is under more attention and evaluation becaue of growing evidence showing that the effects of PTH on cardiac function may be the most serious consequence of secondary hyperparathroism in renal failure.In this study we determined role of excess PTH in the development of left ventricilar (LV) hypertrophy as well as LV ejection fraction in patients with end-stage renal disease under regular hemodialysis. Methods: This study is cross-sectional that was done on patients with end-stage renal disease (ESRD) undergoing maintenance hemodialysis treatment. For patients, Calcium, Phosphorus, Alkaline phosphatase and Intact PTH (iPTH) were measured. Hypertensive patients were stratified into stages one to three. Ecocadiographies for LV hypertrophy and ejection fraction (%) were done and patients stratified into normal ,mild, moderate and severe LV hypertrophy. Results: The total patients were 73(F=28 M=45), consisting of 58 non diabetic hemodialysis patients (F=22 M=36), and 15diabetic hemodialysis patients (F=6 M=9).The mean age was 46.5±16 years. The time on hemodialysis was 21.5±23.5months. The LV ejection fraction (EF%) were 51±8 percent. ‘iPTH’ of patients was 309±349 Pg/ml. ‘iPTH’ of diabetic and nondiabetic groups was 234±265 pg/ml and 329±368 pg/ml respectively. Serum alkaline phosphatase was 413±348 IU/L. Serum alkaline phosphatase of diabetic and nondiabetic groups were 295±179 IU/L and 443±375 IU/l respectively. Serum albumin was 4±0.75 g/dl. Serum albumin of diabetic and nondiabetic groups was 3.6±0.7 g/dl and 4.2±0.7 g/dl respectively. Significant inverse correlation of serum ALP with percent of LV ejection fracction and marginal positive correlation of serum ALP with LVH and also marginal correlation of serum iPTH with LVH were seen. Also significant inverse correlation between serum iPTH with percent of LV ejection fraction in non diabetic heart patients was observed. Conclusions: Adverse effects of secondary hyperparathyroidism on LV function and structure in this study show the role of excess PTH in the development of left ventricilar (LV) hypertrophy as well as low LV ejection fraction in patients with end-stage renal disease under hemodialysis which needs more attention to control of secondary hyperparathyroidism to reduce the risk of cardiovascular morbidity and mortality in dialysis patients .
Keywords: Hemodialysis, Left ventricular hypertrophy, Ejection fraction ,Secondary hyperparathyroidism
Introduction
Cardiovascular disease is the most common cause of death in patients with end-stage renal disease (ESRD) and accounts for much of the morbidity in this population1. Dialysis patients are subject to atherosclerosis and consequent ischemic heart disease, but myocardial dysfunction and overt heart failure also are highly prevalent. Eighty-four percent of patients have left ventricular hypertrophy (LVH), left ventricular (LV) dilatation, or low fractional shortening at the initiation of ESRD therapy, and LVH has been found in 38% of patients with chronic renal failure (CRF) prior to the requirement for dialysis1-2. The presence of LVH or LV dilatation (or both) is clearly a poor prognostic factor2-3. Parathyroid hormone (PTH) is one of the factors that has been implicated in the pathogenesis of a number of cardiovascular abnormalities seen in association with renal failu2-4. Adverse effect of excess PTH on cardiac function was first hypothesized by Selye and by Lehr2. A substantial amount of evidence now exists that suggests a role for excess PTH and the changes in ion regulation induced by PTH in the pathogenesis of uremic cardiomyopathy4,5. A direct effect of PTH on myocardial contractility has not been demonstrated in human adult myocytes, but the cellular influx of calcium induced by PTH has been shown to increase contractility in animal cells2. Indeed, myocardial and vascular cells are a target for PTH via specific receptors on their membranes, experimental studies have shown that PTH produces positive inotropic and chronotropic effects on isolated cardiomyocytes,which occur in association with increased intracellular calcium and cAMP activity4-6. On the other hand, PTH indirectly reduces myocardial contractility2. Albeit, the clinical significance of these effects is not fully undrestood, but, in the terms of LV structural changes, evidences suggests that PTH may play a role in the development of cardiac interstitial fibrosis via the permissive activation of cardiac fibroblasts6-7. There is growing evidence for a role for PTH in the development of LVH.7-9 Cardiac fibrosis is known to be associated with uremia2 and may contribute to diminished LV compliance and consequently diastolic dysfunction in these patients.7-9. In animal models PTH has been shown to activate fibroblasts and to promote the development of intramyocardial fibrosis which is a hallmark of left ventricular hypertrophy in chronic uraemia.6,8-10 Despite the commonly seen abnormalities in serum calcium and phosphate in dialysis patients, only a few studies exist regarding the association between high serum PTH level and left ventricular function and structure in hemodialysis patients, we therefore aimed to consider the evidences regarding the role of excess PTH in the development of left ventricilar (LV) hypertrophy as well as LV ejection fraction in patients with end-stage renal disease under regular hemodialysis .
MATERIAL and methods
This study was carried out on patients with end-stage renal disease (ESRD) undergoing maintenance hemodialysis (HD) treatment. Exclusion criteria were cigarette smoking, body mass index (BMI) more than 25, recent MI and vascular diseases as well as active or chronic infection and pericarditis or pericardial effusion in echocardiography. For patients serum calcium (Ca), phosphorus (P), Alkaline phosphatase (ALP) were measured by standard kits and Intact PTH (iPTH) with DSL-8000 kits by RIA was measured. For stratification of hypertensive patients according to the sixth and seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure we stratified hypertensive patients from stage one to three11,12 (stage of zero equal to no hypertension) stages of the hypertension of HD patients were considered before treatment and at the first start of hemodialysis treatment. For heart echocardiography (2D &doppler ) one single cardiologist who was unaware of the patients data performed all ecocadiographies for determination of left ventricular hypertrophy and left ventricular (LV) ejection fraction (in percent). On the base of sepal thickness we stratified the patients into no LVH (septal thickness between 6-11mm), mild (septal thickness between 11-15mm), moderate (septal thickness between 15-18mm) and severe LVH (septal thickness >18mm). The LVH measurements were done at the end diastolic phase and percent of LV ejection fraction between 55to 75% was considered normal. For statistical analysis descriptive data are expressed as Mean±SD and as frequency distributions. Comparison between groups were performed by using T test. For correlations we used spearmann rho and Partial correlation test after adjustment for age and duration of hemodialysis treatment . All statistical analysis were performed using SPSS (version 11.00) and statistical analysis was significance when p value< 0.05.
Results
The total patients were 73 (F=28 M=45) consisting of 58 non diabetic hemodialysis patients (F=22 M=36), and 15 diabetic hemodialysis patients (F=6 M=9). Tables 1and 2 show the mean± SD of ages, the length of the time the patients have been on hemodialysis and percent of LV ejection fraction as well as lab data, tables 3, 4 and 5 show the frequency distributions of stages of hypertension, chest pain and stages of LVH. The ages of patients were 46.5±16 years. The length of the time patients have been on hemodialysis were 21.5±23.5months. The percent of LV ejection fraction (EF%) were 51±8 percent and 24% of patients had chest pain. Mean±SD of ‘iPTH’ of total patients was 309±349Pg/ml. ‘iPTH’ of diabetic group and nondiabetic group were 234±265 pg/ml and 329±368 pg/ml respectively. Serum alkaline phosphatase of total patients was 413±348 IU/L .Serum alkaline phosphatase of diabetic group and nondiabetic group w ere 295±179 IU/L and 443±375 IU/l respectively. Mean±SD of serum albumin of total patients was 4±0.75 g/dl. Serum albumin of diabetic group and nondiabetic group were 3.6±0.7 g/dl and 4.2±0.7 g/dl respectively. There were no significant difference of age of patients, duration of hemodialysis treatment, serum ALP and serum iPTH between two groups of diabetic and nondiabetic hemodialysis (HD) patients (p>0.05).There was a significant difference of serum albumin between two groups of diabetic and nondiabetic HD patients(p =0.002). Significant difference of percent of LV ejection fraction(EF%) in two groups eas found(47±8 % versus 52±7.8 % respectively)(p =0.026). Significant difference of Ca xP products (46±19 versus 61±24 in non DM-HD patients ) between two groups was seen(p=0.037). In this study there was a significant positive correlation between hypertension with left venricular hypertrophy(LVH) (r =0.606 p<0.001).
Table 1: Mean ±SD , Minimum
and Maximum of age, duration of hemodialysis treatment and LV ejection
fraction.
|
|
Age (years) |
D.H.T* (months) |
LV-EF**, (percent) |
|
|
Total patients
|
Mean±SD |
51±16 |
21.5±23.5 |
51±8 |
|
Min |
15 |
1 |
30 |
|
|
Max |
78 |
112 |
70 |
|
|
Diabetic group |
Mean±SD |
58±16 |
22±23 |
47±8 |
|
Min |
27 |
1 |
30 |
|
|
Max |
78 |
72 |
55 |
|
|
Non-diabetic group |
Mean±SD |
49±15.6 |
21±24 |
52±7.8 |
|
Min |
15 |
1 |
30 |
|
|
Max |
78 |
112 |
70 |
|
*duration of hemodialysis treatment, **LV ejection fraction.
Table 2: Mean ±SD , Minimum and Maximum of laboratory data in hemodialysis patients
|
|
iPTH* (Pg/ml) |
ALP (IU/l) |
CaxP (products) |
Albumin (g/dl) |
|
|
Total patients
|
Mean±SD |
309±349 |
413±348 |
58±24 |
4±0.75 |
|
Min |
10 |
100 |
18 |
2 |
|
|
Max |
2235 |
2438 |
135 |
6.8 |
|
|
Diabetic group |
Mean±SD |
234±265 |
295±179 |
46±19 |
3.6±0.7 |
|
Min |
10 |
120 |
18 |
2 |
|
|
Max |
900 |
734 |
74 |
4.6 |
|
|
Non-diabetic group |
Mean±SD |
329±368 |
443±375 |
61±24 |
4.2±0.7 |
|
Min |
20 |
100 |
25 |
2.5 |
|
|
Max |
2235 |
2438 |
135 |
6.8 |
|
*Intact PTH
Table 3 : Frequency distribution of stages of hypertension (HTN) in hemodialysis patients.
|
Stages of HTN |
Total patients |
DM group* |
Non-DM group |
|||
|
Number |
Percent |
Number |
Percent |
Number |
Percent |
|
|
0 |
7 |
9.6 |
0 |
0 |
7 |
12.1 |
|
1 |
7 |
9.6 |
1 |
6.7 |
6 |
10.3 |
|
2 |
40 |
54.8 |
10 |
66.7 |
30 |
51.7 |
|
3 |
19 |
26 |
4 |
26.7 |
15 |
25.9 |
*DM=Diabetes Mellitus.
Table 4: Frequency distribution of chest pain in hemodialysis patients
|
Chest pain |
Total patients |
Diabetic patients |
Non diabetic patients |
|||
|
Number |
Percent |
Number |
Percent |
Number |
Percent |
|
|
Yes |
24 |
32.9 |
7 |
46.9 |
17 |
29.3 |
|
No |
49 |
67.1 |
8 |
53.3 |
41 |
70.7 |
Table 5 : Frequency distribution of Left ventricular hypertrophy(LVH) in hemodialysis patients
|
|
Total patients |
Diabetic patients |
Non diabetic patients |
|||
|
Number |
Percent |
Number |
Percent |
Number |
Percent |
|
|
No LVH |
12 |
16.4 |
1 |
6.7 |
11 |
19 |
|
Mild LVH |
34 |
46.6 |
7 |
46.7 |
27 |
46.6 |
|
Modrate LVH |
23 |
31.5 |
6 |
40 |
17 |
29.3 |
|
Severe LVH |
4 |
5.5 |
1 |
6.7 |
3 |
5.2 |
Significant linear inverse correlation between hypertension with percent of LV ejection fracction was observed too (r =-0.197 p=0.047). Significant positive correlation between hypertension with Ca x P products of patients (r = 0. 231 p=0.027) was demonstrated also significant inverse correlation between LVH with percent of LV ejection fracction was observed (r = - 0.423 p<0.001).
Partial correlation test after adjustment for ages of subjects , duration of hemodialysis treatment and also serum albumin showed significant positive correlation between serum iPTH and serum ALP(r = 0.302 p=0.005) more over significant linear inverse correlation between serum ALP with percent of LV ejection fracction was observed (r=-0.359 p=0.001) (figure 1) too.
Significant linear inverse correlation between serum iPTH with percent of LV ejection fracction in non diabetic HD patients was found (r=-0.319 p=0.009) (Figure2). Marginal correlation of serum ALP with LVH (r =0171 p =0.050) was observed. Partial correlation test after adjustment for serum albumin and ALP showed significant positive correlation between serum iPTH with LVH (r =0.305 p=0.005) in total patients. Partial correlation test after adjustment for serum iPTH , ALP,age and duration of hemodialysis treatment showed marginal correlation of Ca x P products of patients with percent of LV ejection fracction in total patients (r=0.188 P=0.050) (figure3).
Figure1: Significant linear inverse correlation between serum ALP with percent of LV ejection fraction (r = - 0. 359 p=0.001) (Partial correlation test).
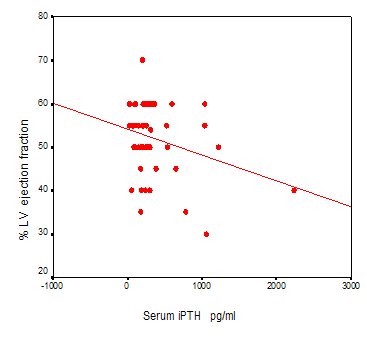 Figure2:Significant
linear inverse co
Figure2:Significant
linear inverse co rrelation
between serum iPTH with percent of LV ejection fracction in non diabetic
HD patients was found too (r = - 0 .319 p=0.009) (Partial
correlation test).
rrelation
between serum iPTH with percent of LV ejection fracction in non diabetic
HD patients was found too (r = - 0 .319 p=0.009) (Partial
correlation test).
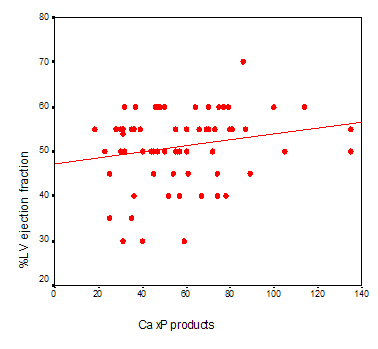 Figure
3: Marginal correlation of Ca x P products of patients with percent of LV
ejection fraction in total patients
Figure
3: Marginal correlation of Ca x P products of patients with percent of LV
ejection fraction in total patients
( r=0.188 P=0.050)( Partial correlation test).
Discussion
The principle findings of this study were positive significant correlation between serum iPTH with LVH, significant inverse correlation of serum ALP with percent of LV ejection fraction, marginal correlation of serum ALP with LVH, and significant inverse correlation between serum iPTH with percent of LV ejection fraction in non diabetic HD patients, marginal correlation of serum iPTH with LVH. Salem in a cross-sectional study in a random sample of 612 hemodialysis patients from 10 dialysis centers examined serum PTH and calcium levels,it was found that 25% of patients had serum PTH levels within the normal range, 25% had a PTH higher than normal (but less than three times normal), and 50% had PTH levels higher than three times normal values, diabetic patients had PTH levels lower than those of nondiabetic patients,results of this study means that hyperparathyroidism are highly prevalent in the hemodialysis population.13 Drueke et al found that correction of severe hyperparathyroidism led to a significant improvement in cardiac performance.14 Timio showed a linear relationship between serum PTH levels and LV mass in dialysis patients.15 Rostand and Drueke reviewed the link between elevated PTH and cardiovascular disease in chronic renal failure patients, they found that elevated PTH was associated with left ventricular hypertrophy and increase left ventriculae mass.16 Kyu-Ha et al. on 62 chronic renal failure patients not yet on dialysis demonstrated that intact PTH was significantly higher in the patients group with LVH compared to without.17 Strozecki et al in a study on 65 HD patients found that LV mass index was lower in normotensive HD patients18. Recently Wanic-Kossowska et al. in 59 HD patients showed positive correlation between PTH serum concentration and LV mass19.Massry has reported an association between exess PTH and a decrease in left ventricular ejection fraction20. Lowrie and Lew21 in a cross-sectional study of more than 12,000 hemodialysis patients,and Foley et al.22 followed 433 patients starting ESRD therapy for an average of 41 months, in both studies, they found that high alkaline phosphatase levels, a marker of hyperparathyroidism, was a significant predictor of death. In an observational study of 189 nondiabetic ESRD patients, Harnett et al. found that an elevated serum alkaline phosphatase, which correlates well with the presence of hyperparathyroidism, was a significant predictor of LVH in a subset of patients on dialysis,for patients with severe LVH , a high alkaline phosphatase was an even better predictor of LVH than was diastolic blood pressure23. In another large cross-sectional study of hemodialysis patients Block et al. showed elevated PTH was a predictor of increased mortality24. In the study of Drueke et al. a significant improvement in cardiac-function was observed after parathyroidectomy , in this research, Drueke, measured various parameters before and 1-2 weeks after parathyroidectomy in 22 hemodialysis patients with secondary hyperparathyroidism,these patients had significant cardiac dysfunction before surgery, with a mean LV ejection fraction of 50.6 ± 2.7% as measured by radionuclide ventriculography,a significant increase in ejection fraction, cardiac index, and myocardial fiber-shortening velocity was observed postoperatively14. Hara et al. in a study on 46 hemodialysis patients ,showed LV impairment was existed in 80% of hemodialysis patients,no correlation between PTH level and LV ejection fraction was existed ,except in a subgroup of patients with an intact PTH level greater than 200 pg/mL,despite this, there was a significant reduction in LV mass and an improvement in LV ejection fraction after parathyroidectomy25,interestingly Nagashima et al. reported A 52-year-old woman, who was a hemodialysis patient that was admitted because of exertional dyspnea, echocardiography showed left ventricular (LV) dilatation and reduced contraction. Coronary angiography showed no fixed stenosis. She had elevated levels of parathyroid hormone as a result of secondary hyperpara-thyroidism with advanced renal failure. After parathyroidectomy, marked improvement of LV function following immediate decrease of blood levels of PTH was observed26. Park et al. could show that treatment of secondary hyperpara-thyroidism with intravenous calcitriol resulted in significant attenuation of myocardial hypertrophy27 In this study we could show the adverse effect of secondary hyperparathyroidism on LV function and structure, as well as in the studies mentioned above, suggest that the effects of PTH on cardiac function may be the most serious consequence of secondary hyperparathroism in renal failure ,elevation of PTH have recently been associated with increase mortality rate among dialysis patients28-29. Regardless of the implications for cardiovascular disease, however, it is accepted that secondary hyperparathyroidism should be controlled to prevent renal osteodystrophy, likewise, serum calcium and phosphate levels should be carefully regulated to assist in PTH control and avoid the complications of an elevated Ca x PO4. these measures may serve to reduce the risk of cardiovascular morbidity and mortality in dialysis patients, In the meantime, further clinical study into this important aspect of the care of ESRD patients is needed.
Acknowledgments
We would like to thank Dr. F. Roghani for echocardiography of the patients.
References
1. Rahn KH, Barenbrock M, Kosch M, Suelak B,Witta J.Vessle wall alterations in patients with renal failure.Hypertens Res 2000;23(1):3-6.
2. Murphy SW, Foley RN. Cardiac disease in dialysis patients, Divalent Ion Abnormalities and Hyperparathyroidism In the Etiology of Cardiovascular Disease of Patients with Chronic Renal Failure. Seminars in Dialysis 1999;12(2):97-101.
3. Zoccali C, Benedetto FA , Mallamaci F, Tripepi G, Giacone G, Cataliotti A et al. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis J AM Soc Nephrol 2001;12: 2768-74.
4. Norris KC. Avoiding the risk of secondary hyperparathyroidism in chronic renal failure:A new approach and a review. Dialysis & Transplantation 2001;30(6).
5. Dyadyk AI, Bagriy AE, Yarovaya NF. Left ventricular hypertrophy in chronic uremia (a review). Dialysis & Transplantation 2000;29(6).
6. Locatelli F, Bommer J, London GM, Martin-Malo A, Wanner C, Yaqoob M et al. Cardiovascular disease determinants in chronic renal failure: Clinical approach and Treatment. Nephrol Dial Transplant 2001;16:459-68.
7. Lopez-Gomez J.M, Jofre R, Cases A. Factores de riesgo cardiovascular en la enfermedad renal cronica. Nefrologia 2002;21:(suppl1).
8. London GM. Calcium-Phosphate disturbances andhyperpara-thyroidism.http://www.sin-italia.org/jnonline/Symposia/ Accord/LONDON/london.html.
9. Rostand SG. Coronary heart disease in chronic renal insufficiency :Some manengement consideration. J Am Soc Nephrol 2000;11:1948-56.
10. Bonisch S, Huge L.U, Amann K, Ritz E. Effect of PTH and ANG II on cardiac fibriblast in vitro. J Am Soc Nephrol 1999;10:616A.
11. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure.Arch Intern Med 1997;157:2413-46.
12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL et al.The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. JAMA 2003;289:2560-71.
13. Salem MM, Hyperparathyroidism in hemodialysis population:A survey of 612 patients. Am J Kidney Dis 1997;29:862-8652.
14. Drueke T, Fauchet M, Fleury J, Toure Y. Lesourd P, Le Pailleure, Crosnier J. Effect of parathroidectomy on left ventricular function in hemodialysis patients. Lancet 1980;i:112-114.
15. Timio M. Cardiotoxicity of parathyroid hormone .It J Mineral Electrolyt Metab 1995;9:19-24.
16. Rostand SG, Drueke TB. Parathyroid hormone ,vitamin D,and cardiovascular disease in chronic renal failure.Kidnet Int1999;56:383-92.
17. Kyu Ha S, Park HS, Kim SJ, Park CH, Kim DS,Kim HS. Prevalence and patterns of left ventricular hypertrophy in patients with predialysis chronic renal failure.J Korean Med Sci1998;13:488-94.
18. Strozecki P, Adamovicz E, Odrowaz-Sypniewska G,wlodarczyk Z, Parathormon MJ. Calcium phosphorus and left ventricular structure and function in normotensive hemodialysis patients.Ren Fail 2001;23(1):115-26.
19. Wanic-Kossowska M, Lehmann P, Czekalski S. Left ventricular hypertrophy in patients with chronic renal failure treated by hemodialysis. Pol Arch Med Wewn 2002;107(6):539-46.
20. Massry SG, Smogorzewski M. Mechanisms through which parathyroid hormone mediates its deleterious effects on organ function in uremia. Semin Nephrol 1994;14:219-31.
21. Lowrie EG, Lew NL. Death risk in hemodialysis patients:The predicting value of commonly measured variables and the evaluation of death rate defferences between facilities.Am J Kid Dis 1990;5:458-82.
22. Foly RN,ParfreyPS, Harnett JD, Kent GM, Hu L, O"Dea R,et al. Hypocalcemia,morbidity and mortality in end-stage renal disease.Am J Nephrol 1996;16:386-93.
23. Harnett JD, Parfrey PS, Griffiths Sm,Gault MW, Barre PE, Guttmann RD. Left ventricular hypertrophy in end-stage renal disease. Nephron 1988;48:107-115.
24. Block GA, Hulbert-ShearonTE, Levin NW, Port FK. Association of serum phosphorus and calcium xphosphate product with mortality risk in chronic hemodialusis patients:A national study. Am J Kidney Dis 1998;31:607-17.
25. Hara S,Ubara Y, Arizono K, Ikeguchi H, Katori H, Yamada A. Relationship between parathyroid hormone and cardiac function in long-term hemodialysis patients. Min Electrolyte Metab 1995;21:67-71.
26. Nagashima M, Hashimoto K, Shinsato T, Ashida K, Kobayashi M, Yamashita H, et al. Marked improvement of left ventricular function after parathyroidectomy in a hemodialysis patient with secondary hyperparathyroidism and left ventricular dysfunction. Circ J 2003;67(3):269-72.
27. Park CW, OH YS,Shin YS. Intravenous calcitriolregress myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroism. Am J Kidney Dis 1999;33:73-81.
28. Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients.Recomendation for a change in manengement.Am J Kidney Dis 2000;35:1226-37.
29. Drueke TB. Aspects of cardiovascular burden in pre-dialysis patients.Nephron 2000;85:9-14.