Introduction
Over the years,
there has been increasing interest in the aetiology of urinary stone formation.
The search has also focused on the detection of specific biochemical
abnormalities of the blood or in urine that would characterize patients with
primary or recurrent urinary calculi. Now, there is convincing evidence that by
treating specific biochemical abnormalities stone recurrence rate can be
reduced.1-6 The treatment measures could either be the "stone
clinic effect" that is fluid plus dietary recommendations 1 or
drug therapy 2-5 and also combination of medical with shock wave
lithotripsy.6 Regular metabolic screening for stone disease is not
practised by all urologists, especially in the first time stone formers;
however the importance of identifying risk factors in the ailment is gaining
recognition.7,8
Before deciding on a
management protocol for our stone disease patients, we investigated for the
presence of biochemical abnormalities in our patients representing a typical
Material and Methods
Over a period of one year and nine months (March 1995 to December
1996), patients with stone disease presenting to the Departments of Urology
& Nephrology at
The blood specimen check included calcium, urate, creatinine, phosphate and bicarbonate, whilst the urine specimen examination included volume assessment and checking the levels of calcium, oxalate, urate, citrate; urinary pH and spot cystine were also checked. Each stone screen cost £35.
A low urinary volume was defined as a volume < 1500 ml / 24 hours in both urinary samples. The normal values for other tests were maintained as determined by the standards of the hospital laboratory.
For statistical analysis, Fisher's Exact test was used.
Results
The total number of patients was 113 including 84 (74%) males and 29
(26%) females. The mean age of patients was 49 (range 14-81) years.
The significant finding of the study was the surprisingly high overall frequency of abnormalities. Out of 113 patients, 83 (73%) had an abnormality in either the blood or urine specimen. Males had 69% (n=58) overall abnormalities, whilst 85% females (n=25) showed abnormalities. This gender difference was however not significant (p=0.0894). The highest number of abnormalities was in the urine.
There was no statistical difference of abnormalities between first time and recurrent stone formers. Females had higher frequencies of low urinary volumes, hyperoxaluria and hypocitraturia. Young patients (<35 years) were 17 (20.5%) in number with a mean age of 28 years (range 17-35 years). The abnormalities were present in 14 (86%) patients in this young age group. The main abnormality in this group was low urinary volume (47%). Family history of stone disease was noted in 2 (12%) of the young group.
Among the urinary abnormalities, the most common abnormality was a low urinary volume noted in 33 patients (39.76%). Exactly the same number of patients had hypercalciuria. The next common abnormality was hyperoxaluria noted in 20 patients (24.1%). Citrate assay was available for only 53 / 83 patients and among these 6 were found to have hypocitraturia (11.32%). This was followed by hyperuricosuria in 8 patients (9.64%). A raised phosphate was noted in just one patient (1.2%). There were no abnormalities of cystine. The summary is given in Figure 1.
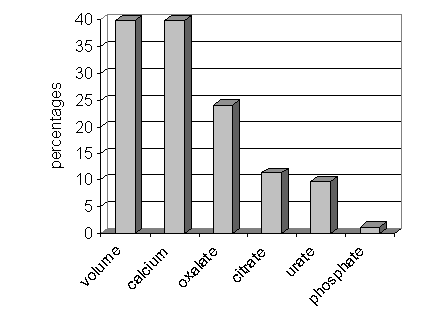
Figure 1: Overall urinary abnormalities in patients as percentages.
The most common blood abnormality noted was a high
serum creatinine in 10 patients (12.05%). Hyperuricaemia was found in 5 patients (6.02%). Only one
patient was found to have hypercalcaemia (1.2%). This
patient was later found to have primary hyperparathyroidism. Similarly only one
person was found to have high serum phosphate who was subsequently also found
to have a high urinary phosphate.
A sub-group analysis to compare the urinary abnormalities between males and females was also performed (Table 1). This showed some interesting features including a high occurrence of low urinary volumes in females, which was 48% (p<0.00 1). The females were also found to have a high incidence of hyperoxaluria at 38% (p<0.002). In addition low urinary citrate was also more common in females at 37% (p<0.001). The male population showed an apparent high rate of hypercalciuria and hyperuricosuria but both these abnormalities were not statistically significant from the similar abnormalities in females.
Table 1: Distribution of urinary abnormalities by gender
Abnormality |
Males (percentages) |
Females (percentages) |
|
Low
volume |
21 |
48* |
|
Hypercalciuria |
30 |
19 |
|
Hyperoxaluria |
11 |
38** |
|
Hyperuricosuria |
7 |
2 |
|
Hypocitraturia |
0 |
37*** |
*p=0.001, **p=0.002, ***p<0.001
In the sub-group analysis of gender related
abnormalities, males had an apparent higher frequency of high
serum creatinine and of hyperuricaemia
but actually this difference between the sexes was statistically not
significant. Similarly, seemingly higher frequency of hypercalcaemia
and hyperphosphataemia in females was not significant
on a statistical analysis from the male patients frequencies of hypercalcaemia and hyper-phosphataemia.
First time stone formers were in the majority at 53 (63.85%) with recurrent stone formers numbering 30 (36.14.7%). Males were in the majority at 73% as first time stone formers and 83% as recurrent stone formers.
Comparative results here show higher frequencies of low urinary volumes (35%), hypercalciuria (23%), hyperoxaluria (17%) and low citrate (10%) in the first time stone formers, but statistically these differences were not significant when compared to the recurrent stone formers.
Whilst recurrent stone formers showed an apparent higher frequency of hyperuricosuria (4%) but this was also statistically not significant when compared to first time stone formers.
Higher frequency of hyperuricaemia (14%) in the recurrent stone formers was statistically not significant from that of the first time stone formers. Equal frequency of raised creatinine of 6% was noted in each group.
Discussion
With better understanding of stone disease pathophysiology9
and impressive results of medical management especially specific treatment,2 the importance of diagnostic evaluation of nephrolithiasis has been realised. 10-12 In
addition, knowing that majority of first time stone formers (FSF) lead to
recurrent stone formation,13-16 significance of early biochemical
investigation can not be ignored. However not every one is very convinced of
its efficacy17 and it is still not practised by all, especially in
the first time stone formers.
The results of our study, by showing a high overall frequency of biochemical abnormalities (73%) in urolithiasis patients and even more surprisingly, almost equal frequencies of various abnormalities between the first time and recurrent stone formers (RSF) has reiterated the importance of diagnostic evaluation, even in patients with first stone episode. One may get an impression from looking at our results that there may have been stone-predisposing dietary habits in our patients, i.e. decreased fluid intake and consumption of foods with a high calcium, oxalate and protein content. The result might also be indicating inadequate medical therapy and random prophylaxis due to the absence of a proper metabolic screening protocol in our practice till recently. Similarity between FSF and RSF abnormalities have also been noted by Yagisawa et al., 18 when low urinary volume and hypercalciuria was identified in both groups but hypocitraturia was more common in the RSF.
Although relatively higher and significant frequencies of low urinary volumes, hyperoxaluria and hypocitraturia were seen in our female patients but as mentioned before the overall female abnormality rate was not statistically higher than males. Higher incidence of hypocitraturia in females has also been reported in other studies. 19-21 However other investigators have not found this sex based difference. 12,22,23
The findings in our study of low urinary volume and hypercalciuria as the main overall abnormalities, followed by hyperoxaluria and hyperuricosuria are well-known stone disease risk factors and reported extensively in the past. A large and relatively recent study quite similar to ours with results also fairly similar to ours was conducted by del Valle et al in 1999. 24
Other risk factors contributing to renal stone disease are also being
identified, which includes plasma phospholipids arachidonic
acid content anomaly25 and female obesity.26
The significance of early
biochemical screening has also been felt by other investigators by highlighting
the cost effectiveness of proper diagnostic evaluation.10,27
Depending upon the severity of stone disease, simple and extensive screening
methods have been described.28 Our method is more similar to the
simpler method already described.
Conclusions
Bearing in mind the above
noted observations based on our study results and knowing that most of the
biochemical abnormalities if treated can considerably lower the recurrence rate
of stone disease, one thus concludes that for rational, efficient and specific urolithiasis management, biochemical screening, particularly urinary screening, should be practised even in
the first time stone disease patients. In addition in some cases of initial
stone presentation, metabolic evaluation can pick up multi-system pathology
responsible for the urolithiasis. Appropriate
treatment of the underlying cause in these cases can therefore prevent extra
renal complications.
REFERENCES
1.
Hosking DH, Erickson SB, Van den Berg CJ, Wilson DM,
Smith LH. The stone clinic effect in the
patients with idiopathic calcium urolithiasis. J Urol 1983;134: 6-10.
2.
Preminger GM,
3.
Laerum E, Larsen S. Thiazide prophylaxis of urolithiasis.
Acta Med Scand 1984; 215: 383-9.
4.
Pak CY, Peters P, Hurt G, Kadesky
M, Fine M, Reisman D et al. Is selective therapy of
recurrent nephrolithiasis possible? Am J Med 1981;
71: 615-22.
5.
Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria
or no metabolic disorder. Ann Intern Med 1977; 87: 404-10.
6.
Fine M, Pak CY, Preminger
GM. Medical management after shock wave lithotripsy. J Urol
1995; 153: 27-32.
7.
Robertson WG. A risk factor model of stone
formation. Front Biosc 2003; 8: s1330-8.
8.
Tiselius HG. Metabolic evaluation and
therapy. Curr Opin Urol 2000; 10(6): 545-9.
9.
Pak CY. Medical management of nephrolithiasis
in
10.
Pak CY, Britton F, Peterson R, Ward D, Northcutt C,
Breslau NA et al. Ambulatory evaluation of nephrolithiasis:
classification, clinical presentation and diagnostic criteria. Am J Med 1980;
69: 19-30.
11.
Preminger GM. Is there a need for
medical evaluation and treatment of nephrolithiasis
in the “age of lithotripsy”? Sem Urol
1994;
12.
Hosking DH, Wilson JW, Liedtke
RR, Smith LH, Wilson DM. Urinary citrate excretion in normal persons and
patients with idiopathic calcium urolithiasis. J Lab Clin Med 1985; 106: 682-9.
13.
Ljunghall S. Incidence of upper
urinary tract stones. Mineral Electrolyte Metabolism 1987; 13: 220-7.
14.
Ljunghall S, Danielson BG. A
prospective study of renal stone recurrences. Br J Urol
1984; 56: 122-4.
15.
Sutherland JW, Parks JH, Coe FL. Recurrence after a
single renal stone in a community practice. Mineral Electrolyte Metabolism
1985; 11: 267-9.
16.
Williams RE. Long-term survey of 538 patients with
upper urinary tract stone. Br J Urol 1967; 35:
416-37.
17.
van Drongelen J, Kiemeney LA, Debruyne F. Impact
of urometabolic evaluation on presentation of urolithiasis: a retyrospective
study. Urology 1998; 52(3):384-91.
18.
Yagisawa T, Chandhoke
PS, Fan J. Metabolic risk factors in first time and recurrent stone formers as
determined by comprehensive metabolic evaluation. Urology 1998; 52(5): 750-5.
19.
Parks JH,
20.
Nikkla M, Koivula
T, Jokela H. Urinary citrate excretion in patients
with urolithiasis and normal subjects. Eur J Urol 1989; 16: 382-5.
21.
Minisola S, Rossi W, Pacitti MT, Scarnecchia L, Bigi F, Carnevale V et al.
Studies on citrate metabolism in normal subjects and kidney stone patients.
Miner Electrolyte Metab 1989; 15(5): 303-8.
22.
Conte A,
23.
Rudman D, Kutner
MH, Redd SC 2nd, Waters WC 4th, Gerron
GG, Bleier J. Hypocitraturia
in calcium nephrolithiasis. J Clin
Endocrinol Metab 1982;
55(6):1052-7.
24.
25.
Baggio B, Budakovic
A, Nassuato MA, Vezzoli G, Manzatto E, Luisetto G et al.
Plasma phospholipid arachidonic
acid content and calcium metabolism in idiopathic calcium nephrolithiasis.
Kidney Int 2000; 58(3): 1278-84.
26.
Powell CR, Stroller ML, Schwartz BF, Kane C, Gentle
DL, Bruce JE et al. Impact of body weight on urinary electrolytes in urinary
stone formers. Urology 2000; 55(6): 825-30.
27.
Preminger GM, Peterson R, Peters PC,
Pak CY. The current role of medical treatment of nephrolithiasis:
the impact of improved techniques of stone removal. J Urol
1985; 134(1): 6-10.
28.
Pak CY. Etiology and
treatment of urolithiasis. Am J Kid Dis 1991; xviii (6): 624-37.