Platelets are small granulated, anucleate cell
fragments circulate as cytoplasmic discs of 6-8fl1. Platelets have a
critical role in the response to injury that involves the process of
hemostasis, thrombus formation, vascular and connective tissue healing2.
Recent studies have revealed a definite role of platelets in the pathogenesis
of atherosclerosis2.
Platelets in contact with damaged or disrupted
endothelium become activated, change their shape from normal discoid shape to
spiny sphere with long thin filopodia, extending several micrometers out from
the platelet and ending in points3. They also release the contents
of their granules during activation. These factors stimulate the proliferation
of smooth muscle cells in the intima of arteries, promote the migration of
fibroblasts from media to the intima of the vessel wall, and aggregate the
activated platelets to one another4.
There have been studies reporting increased platelet
aggregation and increased response to agonists stimulation in vitro5,6,7.
Platelet activation in vivo probably involves a combination of agonists, with
perhaps collagen more important at the beginning, thrombin more important later
on, and with the other agonists in varying mixture throughout8,9.
The present study was designed to evaluate the possible synergistic interaction of subthreshold concentrations of ADP Epinephrine and Epinephrine Collagen in aggregation of human platelets.
SUBJECTS AND METHODS
This experimental study was conducted on 120 samples
of platelets at department of Hematology, Armed Forces Institute of Pathology
(AFIP), Rawalpindi. These samples were isolated from healthy, non-smokers,
non-hypertensive, non-diabetic volunteers who were in age group of 20-50 years
and not on medications for at least last 15 days that is known to interfere
with platelet function.
Subjects were evaluated by taking detailed history,
performing general and physical examination and doing laboratory investigations
like bleeding time, platelet count, blood glucose, serum urea, serum creatinine
and urine for glucose and proteins. All the subjects gave informed consent
before the study.
Platelet Aggregation Studies: Fasting venous blood was collected with minimal venous
occlusion in a plastic conical centrifuge tube containing 3.8% sodium citrate
in a ratio of blood to anticoagulant of 9:1. Precautions were taken to avoid
stasis and contamination with tissue fluids.
The anticoagulated blood in centrifuge tube was
centrifuged at 1500 revolutions per minute (rpm) for 15 minutes at room
temperature. The resultant platelet rich plasma (PRP) was carefully transferred
to a test tube labeled PRP. The remaining anticoagulated blood was
recentrifuged at 4000 rpm for 5 minutes to separate platelet poor plasma (PPP).
A platelet count was preformed on PRP and it was adjusted to 350,000 per
microliter + 50,000 with PRP as needed.
Platelet aggregometer and a chart recorder were
switched on to warm up the heater block upto 37°C and stirring speed was
fixed to 1100 rpm. 500 mL of PPP in a glass cuvette
was placed in a well marked PPP and 450 mL of PRP in another glass
cuvette was placed in a well labeled PRP after adding a magnetic stirring
bar. Platelet aggregation was measured by using a platelet aggregometer
(Chronolog Corporation, USA) which works on turbidometric method described by
Born10, and change in light transmittance was recorded on Omniscribe
Chart Recorder.
The reagents used in the study to aggregate platelets were Adenosine diphosphate in concentrations of 1.0, 1.5, 2.0 and 3.0 mmol/L, Epinephrine in concentrations of 0.3, 0.4, 0.5 and 1.0 mmol/L, and Collagen in concentrations of 2, 3, 5 and 10 mg/ml. after taking the baseline by using its button, the aggregation response was recorded by adding 50 mL of each of the aggregating reagents to each cuvette containing PRP. The aggregation response was interpreted as intensity of aggregation using the technique explained by Roper et al11.
RESULTS
The result of the study showed the mean subthreshold
values of;
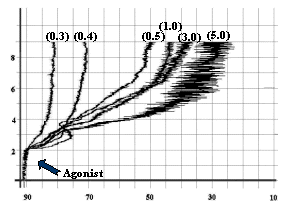
- Collagen as 3 mg/ml, as shown in fig-1.
Fig-1:
Platelet aggregation by different concentrations (mg/ml) of collagen.
![]()
![]()
![]()
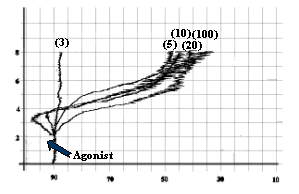
- ADP as 1.5 mmol/L, as shown in
fig-2.
![]()
Fig-2: Platelet aggregation by different
concentrations (mmol/L) of ADP.
![]()
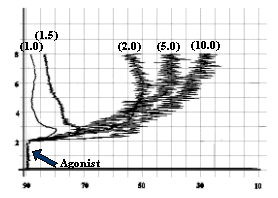
- Epinephrine as 0.4 mmol/L, as shown in
fig-3.
![]()
![]()
Fig-3:
Platelet aggregation by different concentrations (mmol/L) of Epinephrine.
And the combinations of ADP Epinephrine and
Epinephrine Collagen in their subthreshold concentrations showed the
synergistic responses in causing platelet aggregation, as shown in figure-4, 5,
6 and figure 7.
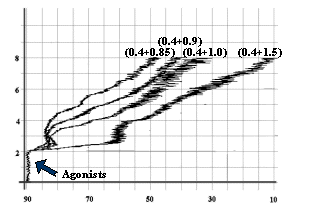
![]()
![]()
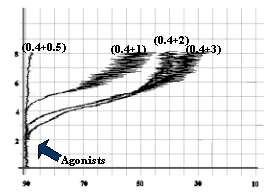
Fig-4: Platelet aggregation by subthreshold
concentration (0.4 mmol/L) of Epinephrine
and subthreshold concentrations (1.5, 1.0, 0.9 and 0.85 mmol/L) of ADP.
![]()
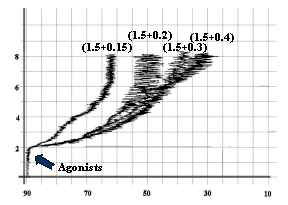
Fig-5: Platelet aggregation by subthreshold
concentration (1.5 mmol/L) of ADP and
subthreshold concentrations (0.4, 0.3, 0.2 and 0.15 mmol/L) of Epinephrine.
![]()
![]()
![]()
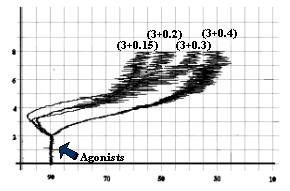
Fig-6: Platelet aggregation by subthreshold
concentration (3 mg/ml) of collagen and
subthreshold concentrations (0.4, 0.3, 0.2 and 0.15 mmol/L) of epinephrine.
![]()
![]()
Fig-7: Platelet aggregation by subthreshold
concentration (0.4 mmol/L) of epinephrine
and subthreshold concentrations (3, 2, 1 and 0.5 mg/ml) of collagen.
DISCUSSION
In the study, only pairs of more common agonists
were studied, since extension to combinations of more agonists would involve a
nearly endless task. We have shown that the aggregation response induced by
epinephrine in combination with ADP was synergistic. Other studies have
demonstrated synergism between these platelets agonists12,13,14.
Ardlie et al15 first reported that epinephrine not only caused
platelet aggregation but also enhanced the aggregation induced by other
agonists, like ADP. Venag et al mentioned the marked enhancement of ADP induced
aggregation on addition of subthreshold concentration of epinephrine16.
The result of present study also showed the
synergistic response in aggregating platelets by epinephrine and collagen when
added together simultaneously in low concentration. The result of this study is
also in agreement with the result of Huang and Detwiler, who demonstrated the
potentiated response to platelet aggregation on combination of collagen and
epinephrine. They also stated that the pattern of responses was intermediate
between that typical for either agonist alone when neither agonist was in
relatively higher concentration17.
Thus, the results of the study revealed that the
optimal platelet aggregation does occur between the above mentioned pairs of
agonists even when each of the agonist is added to the other in subthreshold
doses. Each of these agonists has its own specific receptor on platelet surface
with specific intracellular signal transduction mechanism18,19,20.
As their receptors and associated intracellular signaling pathways in
synergistic responses of these agonists have not been investigated in the
study, this aspect remained unclear.
CONCLUSION
We may conclude in agreement with other
investigators that the synergistic potentiation of some of the agonists in
subthreshold concentrations in vivo may be responsible for the activated state
of platelets and their complications as observed in essential hypertension,
diabetes mellitus, ischemic heart disease, and transient ischemic attacks.
However, the biochemical basis and intracellular signaling of these synergistic
responses needs further exploration.
REFERENCES
1.
Corash
L. The relationship between megakaryocyte ploidy and platelet volume. Blood
Cells 1989; 15: 81-107.
2.
Butt
IF, Aslam M, Khan FA, Ayub M. Plasma insulin and platelet functions in diabetes
mellitus. JCPSP 2000; 10: 182-4.
3.
Nachmias
VT. Platelet and megakaryocyte shape change: triggered alterations in the
cytoskeleton. Sem Hematol 1983; 20: 261-81.
4.
Libby
P, Warner SJ, Salomon RN, Birinyi LK. Production of platelet derived growth
factor-like mitogen by smooth muscle cells from human atheroma. N Engl J Med
1988; 318: 1493-8.
5.
Ishii,
Umeda F, Hashimoto T, Nawata H. Increased intracellular calcium mobilization in
platelets from patients with type 2 (non-insulin dependent) diabetes mellitus.
Diabetologia 1991; 34: 332-6.
6.
Shukla
SD, Paul A, Klachko DM. Hypersensitivity of diabetic human platelets to
platelet activating factor. Thromb Res 1992; 66: 239-46.
7.
Kunisaki
M, Umeda F, Inoguchi T, Watanaba J, Nawata H. Effects of Vit-E administration
on platelet function in diabetes mellitus. Diabet Res 1990; 14: 37-42.
8.
Haung
EM, Detwiler TC. Characteristics of the synergistic actions of platelet
agonists. Blood 1981; 57(4): 685-91.
9.
Kinlough-Rathbone
RL, Packham MA, Mustard JF. Synergism between platelet aggregating agents: The
role of the arachidonate pathway. Thromb Res 1977; 11: 567-80.
10.
Born
GVR, Cross MJ. The aggregation of blood platelets. Nature 1963; 168: 178-95.
11.
Roper
P, Drewinko B, Hasler D, Johnston D, Hester J, Freireich EJ. Effects of time,
platelet concentration and sex on the human platelet aggregation response. Am J
Clin Pathol 1979; 71: 263-8.
12.
Michal
F, Motamed M. Shape change and aggregation of blood platelets: interaction
between the effects of adenosine diphosphate, 5-hydroxytryptamine and
adrenaline. Br J Pharmacol 1976; 56: 209-18.
13.
Mills
DCB, Roberts GCK. Effects of adrenaline on human blood platelets. Am J Physiol
1967; 193: 443-53.
14.
Osmani
AH, Clare KA, Scrutton MC. Synergistic interaction and platelet inhibitory
agents. Thromb Res 1983; 31: 665-74.
15.
Ardlie
NG, Glew G, Schwartz CJ. Influence of catecholamine on nucleotide-induced
platelet aggregation. Nature 1966; 5060: 415-7.
16.
Vanags
DM, Rodgers SE, Duncan EM, Lloyd JV, Bochner F. Potentiation of ADP induced
aggregation in human platelet-rich plasma by 5-hydroxytryptamine and
adrenaline. Br J Pharmacol 1992; 106: 917-23.
17.
Huang
EM, Detwiler TC. Characteristics of the synergistic actions of platelet
agonists. Blood 1981; 57: 685-91.
18.
Ware
JA, Smith M, Salzman EW. Synergism of platelet-aggregating agents. J Clin
Invest 1987; 80: 267-71.
19.
Hallam
TJ, Scrutton MC, Wallis RB. Synergistic responses and receptor occupancy in
rabbit blood platelets. Thromb Res 1982; 27: 435-45.
20.
Smith
JB, Selak MA, Dangelmaier C, Daniel JL. Cytosolic calcium as a second messenger
for collagen-induced platelet responses. Biochem J 1992; 288: 925-9.