INTRODUCTION
The function of Leydig cells of the testis is to secrete androgens in a
regulated fashion. The principal regulating mechanism consists of the secretion
of pulses of luteinizing hormone (LH) by the
adenohypophysis1,2.It has long been known
that cyclic AMP accelerates the synthesis of androgens by Leydig
cells3 and that LH increases the levels of cyclic AMP in these cells4.
In view of the extensive evidence that cyclic AMP mediates the actions of
various hormones, it has been concluded that the responses of Leydig cells to LH and hCG result
from increased production of cyclic AMP as the result of the binding of LH to
its receptor5.
b-Adrenergic
receptor antagonists (b blockers) have
received enormous clinical attention because of their efficacy in the treatment
of hypertension, ischemic heart disease, and certain arrhythmias. Ahlquist's hypothesis that the effects of catecholamines were mediated by activation of distinct a- and b-adrenergic
receptors provided the initial impetus for the synthesis and pharmacological
evaluation of b-adrenergic
blocking agents6. Atenolol (Tenormin) is a b1-selective
antagonist that is devoid of intrinsic sympathomimetic
activity. Atenolol is very hydrophilic and appears to
penetrate the brain only to a limited extent. Its half-life is somewhat longer
than that of metoprolol7.
Along with
increasing use of various b-1- selective
adrenergic antagonists in medical practice, a growing number of reports have
emphasized the risk of sexual side effects8. Expanding information
concerning their effect on adult male fertility will be of benefit to
physicians, investigators and their patients.
MATERIAL AND METHODS
Two Wistar
male rats, weighing 190± 10 g about 90 days
old were taken per experiment. These rats were bred at animal house of Aga Khan University Karachi under standard conditions with
a daily photo period of 16 hours light: 08 hours dark at 23°C. The rats had
free access to food and water ad libitum.
The testes were
dissected out and decapsulated. After decapsulation 4 testes were put in 10 ml of Eagles Medium
199 containing 0.25-mg/ml collagenase. This was
incubated at 37° C for 25 minutes
with a constant shaking at 100 cycles/minute in long axis parallel to the
direction of the movement. 20 ml of cold saline was added to stop incubation
process. Filtered portion was centrifuged at 80 g for 10 min at 4° C to remove collagenase.
The cell suspension
was preincubated for an hour to remove any endogenous
production of testosterone at 34°C. After
pre-incubation, cell suspension was centrifuged for 10 min at about 200 g. The
pellet was resuspended in incubation medium to give
85,000 viable cells per 200 ml 9.
Equal amounts of both Trypan
blue (0.1%) and cell suspension were taken (30 ml each) for cell counting in Neubauer
Chamber (WBC) (73). At least 85000 cells/200 ml were taken.
The rat Leydig cells were incubated with varying concentrations of Atenolol: [Selective Beta-Adrenergic Antagonist} (10-6,
10-7 and 10-9 M) with or with out LH 250 IU for three
hours to measure the testosterone release.
Testosterone was measured by
radioimmunoassay (RIA) according to a WHO protocol, and regents were supplied
through the WHO Matched Regent Programme.
Testosterone was measured in extracted samples, whereas RIA reagents were
directly added to tubes containing incubation medium without application of any
extraction procedure. After addition of all the reagents, tubes were incubated
overnight (28-24 h) and the bound fraction was separated from the unbound by
the addition of 1% Dextran coated charcoal.
Testosterone concentrations were calculated by logit-log
transformation using a computer programme.
Statistical Package for Social Sciences Version 7.5
(SPSS 7.5) analyzed the data. The differences between the control and the
treated samples were calculated by Student’s “t” test. The arithmetic means and
standard deviation were calculated for both samples separately.
The Confidence Interval was 95%. The values were
considered significant when P<0.05 whilst these were labeled non significant
when P>0.05 as compared to the levels observed in the control group.
RESULTS
The basal release of
Testosterone release was 37.15±0.25 pg per tube Administration of LH 250 IU
increased the basal release of Testosterone significantly (P<0.001) which
mounted to 211.43±6.62 pg/tube.(Fig 1)
Release of
Testosterone under the effect of Atenolol 10-6
was 33.76±0.25 pg per tube, which was statistically significant (P<0.05)
decrease as compared to basal release of 37.15±0.25 pg per tube. Atenolol at
concentrations of 10-7 and 10-9 also did not exhibit any
significant effects on Testosterone release.(Fig 2)
Administration of
LH 250 IU increased the basal release of Testosterone significantly
(P<0.001) up to 211.43±6.62 pg per tube. When LH 250 IU and varying
concentrations of Atenolol were administered
together, it was observed that Atenolol 10-6
and 10-7 caused a significant decrease in Testosterone levels
(120.64 ±0.35 and 155.09 ±2.20 pg per tube) as compared to the levels of
testosterone produced alone by LH. (P<0. 01). While Atenolol 10-9
concentration along with LH 250 IU was unable to have significant effect.(Fig
3)
DISCUSSION
In the adult males,
LH acts at multiple levels to stimulate steroidogenesis
and to maintain normal Leydig cell function. In
vitro, LH exerts immediate effects on protein synthesis, protein phosphorylation, and steroid synthesis, and has long-term
effects on transcription of the steroidogenic enzymes
and the intracellular structures important for steroidogenesis.10
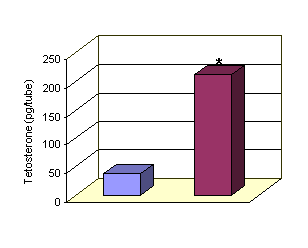 Control LH 250 IU
Control LH 250 IU
*LH 250 IU vs control P<0.001
Figure 1: Effect of LH on Testosterone release by Leydig cells.
A t e n o l o l
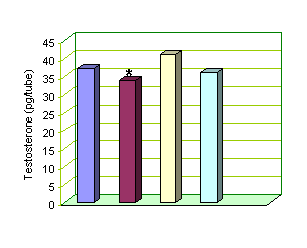 Control 10-6 M 10-7 M 10-9 M
Control 10-6 M 10-7 M 10-9 M
* Atenolol 10-6 vs control P<0.05
Fig-2: Effect of varying concentrations of Atenolol on Testosterone release by Leydig
cells.
In the absence of LH in vivo,
there is a rapid decline in Testosterone secretion by the Leydig
cell and a gradual regression of the Leydig cells
with loss of cytoplasmic volume and the intracellular
structures associated with steroidogenesis, although Leydig cell numbers are marginally affected.11
In our study when Leydig
cells were incubated with LH 250 IU for three hours it was observed that there
was a significant (P<0.001) rise in the Testosterone release as compared to
the basal release of Testosterone. The key steps of the steroidogenic
pathway which are acutely regulated by LH action are the mobilization of stored
cholesterol, transport of cholesterol into mitochondria and the resulting
activity of the cholesterol side chain cleavage complex 2.
LH 250 IU with Atenolol
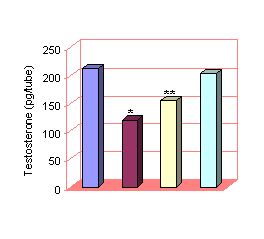 LH 250 IU 10-6
M 10-7 M 10-9 M
LH 250 IU 10-6
M 10-7 M 10-9 M
* LH with Atenolol 10-6 vs LH
250 IU P<0.001
** LH with Atenolol 10-7
vs LH 250 IU P<0.01
Figure 3: Combined effect of LH and varying concentr-ations of Atenolol on
Testosterone release by Leydig cells
It is well recognized that the administration of
beta-blockers to patients after myocardial infarction improves their survival
rate 12. Review of previous usage of beta-blockers and of
contraindications along with the current analysis of a uniform discharge
summary has resulted in a significant increase in the use of beta-blockers as
life saving drugs 13.
In the current study we observed that when Leydig cells were incubated with beta-1 selective
antagonist: Atenolol, in a varying concentrations (10-6,
10-7 & 10-9 M) caused significant (P<0.05)
reduction in Testosterone release by the rat leydig
cells as compared to the basal release of Testosterone in a dose-dependent
fashion. This decrease in the Testosterone release by the Leydig
cells under the effect of Atenolol seems to be the
main cause of sexual dysfunction experienced by patients taking beta-blockers.
When Leydig cells were
incubated with both LH 250 IU and varying concentrations of Atenolol
(10-6, 10-7 & 10-9 M), it was seen that
the Testosterone release by the Leydig cells was
significantly lower (P<0.001) as compared to the Testosterone release by the
Leydig cells when incubated alone with LH 250 IU,
again in a dose-dependent manner. Atenolol decreased
the Testosterone release by LH-stimulated Leydig
cells more significantly as compared to the effects of Atenolol
produced on non-stimulated Leydig cells.
In a study Forgari et al has reported that Atenolol
induces worsening of sexual activity and reduction of testosterone in
hypertensive patients 14.
It is concluded that Atenolol
causes a reduction in Testosterone release in a dose-dependent fashion both in
non-stimulated and LH stimulated rat Leydig cells.
REFERENCES
1.
Dufau ML, Veldhuis J, Fraioli F, Johnson
MH, Catt KJ. Mode of bioactive LH secretion in man. J
Clin Endocrinol Metab 1983; 57:93-1003.
2.
Hedger MP, de-Kretser-DM.
Leydig cell function and its regulation. Results-Probl-Cell-Differ 2000; 28 :69-110.
3.
Sandler R, Hall
PF. The influence of age upon the response of rat Biophys
Acta 1968; 164:445-51.
4.
Mellon SH, Vaisse
C. cAMP regulates P-450scc gene expression by a cycloheximide insensitive mechanism in cultured mouse Leydig MA-10 cells. Proc Natl Acad Sci
5.
Yano-K. The functional analysis of
LH receptor. Nippon-Rinsho. 1997; 55 (2): 487-90.
6.
Pujet JC, Dubreuil C, Fleury B, Proviedier O, Abella ML. Effects
of celiprolol, a cardioselective
beta blocker, on respiratory function in asthmatic patients. Eur. Respir. J 1992; 5:196-200.
7.
Brodde OE. The
functional importance of beta1 and beta2 adrenoceptors
in the human heart. Am J Cardiol. 1988; 62:24C-29C.
8.
Wadworth AN, Murdoch
D , Brogden RN. Atenolol. A
reappraisal of its pharmacological properties and therapeutic use in
cardiovascular disorders. Drugs 1991:42:468-510.
9.
Abdul Saeed
S, Zaidi AA , Pertani SA.
Effect of chronic treatment with cyclooxygenase
inhibitor (indomethacin) on the pituitary-testicular
axis. Med Sci Res; 1995:
23: 85-7.
10.
Dufau ML, Miyagawa TF, Takada S, Khanun A, Miyagawa H, Boczko E. Regulation
of androgen synthesis: the late steroidogenic
pathway. Steroids 1997;62:128-32.
11.
Duckett RJ,
Hedger MP, McLachlan RI, Wreford
NG. The effects of gonadotrophin-releasing
hormone-immunization and recombinant follicle-stimulating hormone on Leydig cell and macrophage populations of the adult rat
testis. J Androl 1997;18:417-23.
12.
Kizer KW, Sawin CT. Increased use of beta-blockers after acute
myocardial infarction. Am J Med 2000; 108(4): 349-50.
13.
Dall L,
Simmons T, Peterson S, Herndon B. Beta-blocker use in patients with acute
myocardial infarction treated by hospitalists. Manag Care Interface 2000; 13(5): 61-3.
14.
Fogari R, Preti P, Derosa G, Marasi G, Zoppi A, Rinaldi A, Mugellani A. Effect of
antihypertensive treatment with valsartan or Atenolol on sexual activity and plasma testosterone in
hypertensive men. Eur J Clin
Pharmacol 2002;58 (3): 177-80.