INTRODUCTION
Acute
myeloid Leukemia is a heterogeneous disease. Therefore parameters are needed to
classify this disease into biologic entities to understand its pathogenesis and
develop specific treatment approaches.1, 2 As therapeutic advances
were made, distinguishing the subtypes of acute leukemia became increasingly
important.
Acute
leukemias are classified on the basis of the presumed cell of
origin.Concordance between experienced observers in the classification of acute
leukemia increases from 70 to 99% when morphologic criteria are supplemented by
cytochemical and immunphenotypic information.3
The modern era for classification of acute leukemias
dates back to 1976, when international group of investigators from
The FAB classification of AML divides cases into eight
major groups with subtypes for three of them (table 1). The classification
criteria are based on morphologic and cytochemical features; however for some
of the categories, immunophenotyping is necessary.6, 7 It is
lineage-based morphological classification that categorizes cases according to
the degree of maturation of the leukemic cells and their lineage
differentiation. The major advantage of the FAB classification system is its
ease of use. The cytological criteria are well defined; they do not require
high technology and can be applied in most laboratories through out the world.
8 Keeping in view these advantages, the FAB proposal was adopted
internationally. It provided long needed standard terminology and was quickly
accepted by most of the multi-institutional study groups for management plans
and comparison of treatment results between morphologic subtypes for their
prognostic significance.
Present study was done to determine the frequency of
AML types in our population. As the patients were from all over
Although, it is a single institution based study, the
number of cases studied is largest that is ever reported at national level.
Hence, our study tends to establish a trend of AML subtypes in
Material and methods
This
is descriptive case-control study done at hematology department of
Patients with history of previous hematological
disorders like myelodysplasia, CML, aplastic anaemia and with history prior
chemotherapy or radiotherapy were excluded from the study. The patients who
were known cases of AML with or without treatment including relapsed cases were
also not included.
Table-1: FAB Classification of AML
|
Myeloblastic leukemia
minimally differentiated
M0 Myeloblastic leukemia
without maturation
M1 Myeloblastic leukemia with maturation M2 Hypergranular promyelocytic leukemia M3 ·
Microgranular variant Myelomonocytic leukemia M4 ·
With bone marrow eosinophilia M4Eo Monocytic Leukemia
M5 ·
Poorly differentiated M5a ·
Differentiated M5b Erythroleukemia
M6 Megakaryoblastic Leukemia
M7 |
Based on this, a total of 116 subjects with newly
diagnosed untreated denove AML were included in the study. It included patients
of all age groups and both sexes.
The diagnosis of AML was established according to the
standard practice, and was based on peripheral blood and bone marrow morphology
and cytochemistry. Immunophenotyping was done where considered essential.
Hematological parameters were done on Coulter counter (model stak-s).
Bone marrow aspiration was done from posterior iliac
crest. A written consent was taken from patients or parents as appropriate. In
every case, 6-8 smears were made; two of them with peripheral smears were
stained by Leishman’s stain. In addition following cytochemical stains were
carried out on peripheral blood and bone marrow smears in each case:
All the cases were reviewed by first and third author
independently. On the basis of morphology, cytochemistry and immunophenotyping,
AML was classified into its various subtypes. (M0-M7) using FAB criteria. (Table-1)8.
The presence or absence of Auer
rod in each bone marrow film was also noted.
RESULTS
Of
116 cases, 70 were males and 40 were females with male to female ratio1.5:1.
The mean age was 32 years (range 6 months –85 years). 21 cases were up to the
age of 15years comprising of 17 males and 4 females and their age ranges
between 6 months- 15 years. Their haematological parameters are given in table
2. The CBC showed a wide range of variation in hemoglobin concentration and
platelets ranging from subnormal to normal. Their Leukocyte count also showed
variation from leucopenia to hyper leukocytosis.
In our study, we found AML (M4 FAB subtypes) to be the
commonest comprising 42 out of 116 of total cases (36.25%). This also included
6 cases of M4Eo variant. The frequency of various AML subtypes according to FAB
classification is given in Figure 1.
Figure 1: French –American
–British (FAB) Sub types in 116 cases of AML
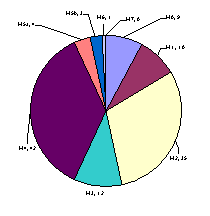
The cases were further divided in adult and children
group. Both groups revealed M4 as a most common FAB type followed by M2.
Relative frequencies of various subtypes for adults and children are
illustrated in Table 3.
Auer rods were present in 36.2 %( 42 cases). They were
more common in M3 subtype (9/12, 75%) followed by M2 (18/35, 51.4%), M4 (12/42,
28.5%), M1 (2/10, 20%) and M5 (1/7, 14.2%).The number of Auer rods per cell was
consistently greater in M3 subtype. No Auer rods were observed in the M0 or M6
subtype.
Table-2: Haematological parameters in AML patients (n
= 116)
|
|
Mean |
Range |
|
Hemoglobin
(gm/dl) |
8.4 |
2.2-14.4 |
|
White
Cell Count (109/l |
63.39 |
0.6-497.4 |
|
Platelet
count (109/l) |
48.9 |
1.0-270.0 |
Table 3: FAB distribution of AML according to age group
(n= 116)
|
Fab Group |
< 15
Years n % |
> 15
Years n % |
|
M0 |
05 (23.8) |
04 (4.2) |
|
M1 |
01 (4.7) |
09 (9.4) |
|
M2 |
06 (28.5) |
29 (30.5) |
|
M3 |
00 (0) |
12 (12.6) |
|
M4 |
07 (33.3) |
35 (36.8) |
|
M5
a |
00 (0) |
04 (4.2) |
|
M5
b |
01 (4.7) |
02 (2.1) |
|
M6 |
01 (4.7) |
00 (0) |
|
M7 |
00 (0) |
00 (0) |
|
Total |
21 (100) |
|
DISCUSSION
With
the introduction during the late 1960s and 1970s of increasingly effective
therapy for acute leukemias, it became necessary to determine subgroups, which
might require different treatment approaches. In 1976 FAB system 4
of classification was introduced, which was subsequently revised at various
times to improve concordance.5, 6, 7 This system provided structured
criteria for the diagnosis of various sub types of AML and is based mainly on
morphological and cytochemical features; for some of the categories,
immunophenotyping is necessary.8,9 Since than it has been widely
adopted nationally and internationally for classification of acute leukemias,
although ambiguities still give rise to confusion and it has been the subject
of recent criticism.10 So, in 1997 a revised WHO classification of
AML was published which included cytogenetic studies as well.11 However this classification is not practiced
widely at national level because of financial constraints. The clinical and
biological significance is also claimed for this system which accounts for
distinct prognostic differences for various subtypes and their close
association with chromosomal abnormalities. Although FAB proposals may be considered over
simplification, but they do serve to provide the guidelines for both
hematologists and non specialists and facilitate quick, consistent diagnosis
and classification of these diseases. It is for these reasons that FAB is still
favourite and popular among Pakistani hematologists.
The FAB distribution of AML has been extensively
studied in the past decades at national and international levels.9,12-19
In table 4 the adult results are compared with other series using FAB system.
Most published data indicate the predominance of M2 as a most common subtype.9.14-16,18
Occurrence of this subtype is also common after primary malignancy.20
However, two studies from Saudi Kingdom reported predominance of M4
and
M5
subtypes.13,17 Nakase et al
showed AML-M4 as common subtype in Australian population compared to Japanese,
where AML-M2 is common.21 Present study also confirms M4 as the most
common type followed by M2. This is in concordance with the previously
published results from our institution by Kakepoto et al.19
Many of the differences in AML subtypes may be due to
the subjectivity of morphologic diagnosis together with variable nature of
acute myeloid leukemia subtypes, with no real demarcation. Some genetic factors
may be responsible for a particular FAB subtype of AML in our population.
Secondly most studies at national level have small number of patients and
probably with underutilization of cytochemical stains. Moreover these studies
were not subjected to immunophenotyping that may be because of error in
diagnosis. The other reason for this discrepancy may be patients of different
ethnic group and/or geographical variation.
Auer rods were seen in 36% of cases with highest
frequency in M3. Spence et al 13 reported Auer rods in 40.6% of
cases in their series. These results are consistent with present study.
Male to female ratio in present study is 1.5:1, which
is in concordance with national and international studies.14,16,17,22 The mean age (32 years) at presentation seems
to be lower than the expected mean age reported in western countries where AML
peaks in incidence after the 6th decade of life.23
However, this is similar to mean age reported in studies from Saudi Arabia 17
and Pakistan.16,19,24 The difference of mean age between
present study and western studies is probably due to different geographical
distribution.