DIFFERENCES BETWEEN MALE AND
FEMALE STUDENTS IN CARDIOVASCULAR AND ENDOCRINE RESPONSES TO EXAMINATION STRESS
Mohammad Khaksari1, Mehdi Mahmoodi2, Mohammad
E. Rezvani3, Mohammad A. Sajjadi4, Gholamreza. Asadi
Karam2, Sohrab Hajizadeh5
1.Dept. of
Physiology and Pharmacology, Faculty of Medicine,
Kerman University of
Medical Sciences, 2. Dept. of Biochemistry and Biophysic, Faculty of Medicine,
Rafsanjan, 3. Dept. of Physiology, Faculty of Medicine, Rafsanjan, 4. Dept of
Internal Medicine, Faculty of Medicine, Rafsanjan, 5. Dept. of Physiology,
School of Medical Sci. Tarbiat Modarres University, Tehran, Iran.
Background: It is known that stress alters biological
processes. The aim of the present study
was to examine the effect of examination stress in young adult male and female
students. Methods: Examination stress was studied in 28 young female and
21 young male volunteer students of Rafsanjan university of medical Sciences,
0.5 hour before Physiology examination (stress condition) at 10-12 a.m. and 45
days after examination (control condition) at the same time in the year 2003. Results:
There were no differences in BMI of male and female groups at control and
stress conditions. Subsequent analysis
between two sexes showed that males had significantly higher systolic [SBP
(124.7±4.01 mmHg)] and diastolic blood pressure [DBP (76.56±2.48 mmHg)], heart
rate [HR (84.6±2.63)] increases in stress condition, in both sexes, but in
males the increasing of HR is more than females, whereas females had higher
repsiratory frequency increase in stress condition, compare to males. Moreover, there were no differences in SBP,
DBP and HR responses to stress condition in different phases of the menstrual
cycle. The increased amonut of the
plasma cortisol in stress condition was significantly higher in males
(485.3±37.9 in stress vs. 335.7±27.9 pg/ml in control) than females, stress
also reduced females’ ACTH in both phases of the menstrual cycle (13.3±0.8 in
stress vs. 27.47±7.25 pg/ml in control), but in males stress increased ACTH
(43.72±4.45 in stress vs. 49.29±3.25 pg/ml in control). In males, stress induced a significant
decrease in plasma testosterone. Plasma
progesterone in response to stress showed a significant decrease in the luteal
phase. Conclusion: These data suggest that, the responses to
physiology examination stress are different between two sexes.
Key words: Cardiovascular; Endocrine; Physiology Examination; Sex
differences; Stress.
INTRODUCTION
Stress is the specific and
nonspecific response of the body to any kind of physiologic pressure or
unwanted forces due to environmental or peripheral effects. Stress acts in different axes including
hypothalamus-pituitary–adrenal (HPA) axis, hypothalamus–pituitary–gonads (HPG)
axis, and hyphothalamus–pituitary–thyroid (HPT) axis.1,2 In addition, there are autonomic responses by
sympathetic nerve system to the stressful condition i.e. the activation of
sympathetic nerve system and the activation of the above axes specially HPA occur,
simultaneously.2,3 Many kinds
of different stress need the responses of endocrine glands, for example
responses to the stress of temperature, hypoglycemia, surgery, pain,
occupations, or decreasing energy, are mediated by different endocrine
glands. Individual diffrences in
cardiovascular and neuroendocrine responses to stress are cardiovascular
complication (hypertension and increasing heart beat), gastric ulcer, migrane
headache, and asthma.2
Among
neuroendocrine stress reactions the releasing of catecholamines, adrenaline and
noradrenaline play a key role in human adjustment to environmental demands, for
example, augmentation of the peripheral catecholamines level is accompanied by
a series of changes in cardiovascular and metabolic function which facilitate
adaptation to a wide range of stimulus conditions.2
Human studies
suggest that cardiovascular responses to stress are sex- dependent and in
females differ during the menstrual cycle.4,5 Women usually have
lower blood pressure and adrenaline in responses to stress than do men,6 suggesting
that determinants of women’s stress responses may differe from men’s
responses. It has been shown that
premenopausal and postmenopausal women differed in physiological responses to
behavioral stressor.7
Most of the
studies in different responses to stress carried out in middle age men and
women, and there were not many reports that investigated the effects of stress
on the young university students. The
examination stress was accounted as an acute physiologic stress in some
subjects. Examinations are anecdotally
viewed as extremely stressful to Singapore schoolchildren.8 Patients with acne may experience worsening
of the disease during examinations.9 A study in Netherland on PhD
students showed that although the blood pressure did not changed significantly
by the examination stress, but peripheral benzodiazepine receptor density,
allopregnanolon, and cortisol concentration were significantly increased during
examination.10
It is crucial to
survey the determinants of young male’s and female’s stress induced by
examination, therefore, the general aim of this study was: firstly, to measure
the cardiovascular and neuroendocrine responses to examination stress in young
students, secondly, to find whether the responses are sex dependent, and
thirdly, is there any differences in the responses to stress in follicular and
luteal phases of menstrual cycles.
MATERIAL
AND METHODS
Examination stress was studied in
28 young females and 21 young males, 19-23 year old of Rafsanjan medical
volunteer students, and there was no difference in age between males (21.5±1.3
years) and females (20.4±1 years). Mean
value for weight and height for males, were 59.35±3.86 kg and 175±1.4 cm
respectively, which significantly were greater than those were for females,
54.78±1.21 kg and 163.56±0.97 cm respectively.
Students were asked to fill in a questionaire for the stressful
examinations and specify which subject is more stressful. After reviewing the questionaires it was
clarified that physiology examination is among the stressful subjects. Then for the control of stress condition and
elimination the interfering factors, all of the individuals who had other
stresses, except the examination i.e. psychological problems or took medicines
were withdrawn from the study.
The effect of stress was
investigated half an hour before final physiology examination in January 2003
(stress condition) and 45 days after examination (control condition, ordinary
university work) in Feberuary at 10-12 a.m.
The body mass
index (BMI), systolic and diastolic blood pressure (SBP and DBP respectively),
heart rate (HR), respiratory freqeuncy, cortisol, estradiol, progesterone,
testosterone and ACTH were measured at control and stress conditions. Blood
pressure and heart rate were measured using sphygmomanometer and
stethoscope. Blood samples were taken 30
minutes before examination and 45 days after examination at 10-12 a.m. The
cortisol, ACTH, progesterone and testosterone of blood plasma were measured by
radioimmunoassay (RIA). To determine the
follicular or luteal phase, the females were asked how many days were passed
from their last menstruationcyle and
that was confirmed by the measurement of blood progesterone concentration.
All
subjects had their informed written consent for participation in the
study. The experimental design and the
procedures followed were in accordance with the ethical standards laid down for
human studies.
Data
were analysed by analysis of variance (ANOVA) to compare the differences
between groups, and to determine the differences between two groups the Tukey
test were used. The data are presented
as Mean±SEM and P<0.05 was considered as statistically
significant.
RESULTS
The
results of this study are summarized in tables 1 and 2 and Figures 1-5.
Table 1
summarises some demographic charactristics of the female and male
students. Both weight and height of
males are significantly more than females (P<0.001), but there was no
significant difference in BMI between two sexes.
Table 2 shows the mean
of systolic and diastolic blood pressure (SBP and DBP respectively), heart rate
(HR) and respiratory frequency (RF) for male and female students in stress and
control conditions. As data presents SBP
is significantly higher in males than females (P<0.01). For males SBP is
significantly greater in stress than control condition (P<0.05). DBP in
stress condition increased in male compare to female significantly (P<0.01).
The HR for
females in stress condition is
significantly increased compare to control; this is the same for males. In control condition HR for females is
significantly greater than males (P<0.001), therefore for males there is a
higher increase of HR, than females in stress condition. RF for female was more than males in both
control and stress conditions (P<0.05).
Table-1: Selected
demographic charactristics of the female and male students in the physiology
examination stress and control condition.
Data reperesents mean±SEM.
|
Variable
|
Conditions
|
|
Control
|
Stress
|
|
Female
|
Male
|
Female
|
Male
|
|
Weight(kg)
|
54.78±1.21
|
***59.35±3.86
|
55.6±1.2
|
***66.68±1.8
|
|
Height (cm)
|
163.56±0.97
|
***175×1±1.4
|
164.32±0.97
|
***175.6±0.98
|
|
BMI(kg m-2)
|
20.4±0.4
|
21.52±0.46
|
20.56±0.45
|
21.84±0.57
|
|
Age(year)
|
21.4±1.5
|
22.5±1.75
|
21.7±1.4
|
22.9±1.65
|
***: Significant
sex difference, P<0.001. n=28 for
females and 21 for males.
Table-2: Mean and standard
errors for systolic (SBP), diastolic blood pressure (DBP), Heart rate (HR), and
respiratory frequency (RF) in female and male students under physiology
examination stress and control conditions.
Data repersents Mean±SEM
|
Variable
|
Conditions
|
|
Control
|
Stress
|
|
Female
|
Male
|
Female
|
Male
|
|
SBP (mmHg)
|
112.34±1.6
|
b116±2.7
|
110.8±2.25
|
a 124.76±4.01
|
|
DBP (mmHg)
|
67.96±1.27
|
74.2±1.9
|
68.22±2.03
|
c 76.56±2.48
|
|
HR (beat min-1)
|
77.09±1.04
|
70.2±1.64
|
d 84.9±1.25
|
84.6±2.63
|
|
RF (beat min-1)
|
18.48±0.28
|
16.88±0.21
|
f 20.81±0.52
|
17.12±0.28
|
a:
Significant sex difference under stress condition, P<0.01, b: Significant difference in males
under stress and control condition, P<0.05, c: Significant sex difference under stress condition, P<0.05, d: Significant difference in females
under stress and control condition, P<0.001, f: Significant sex difference under stress condition, P<0.001,
n=28 for females and 21 for males.
The salient results have been
summarized in figures 1-5. Figure-1 shows plasma ACTH concentration in females (luteal and follicular phases) and males under control and
stress conditions. Figure-2 illustrates females’ cortisol
mean concentration in control and stress condition in both phases and the
comparison of their serum cortisol with males.
Figure-3 represents males’ serum testosterone mean concentration in control and
stress condition. Figure-4 shows the mean concentration of estradiol
in females’ follicullar and luteal
phase, while the mean concentration of progesterone in females at both phases
is shown in figure 5.
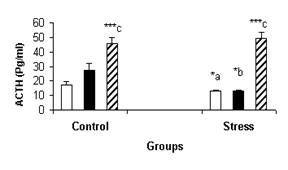
Figure-1: Mean ± SEM of ACTH plasma
levels during stress and control conditions in males (▒), follicular (c) and luteal (g) phases. a: significant difference
in follicular phase under stress compare to control. b:
significant difference in luteal phase under stress
compare to control. c: significant sex difference
between males and females, in the stress and control conditions.*: P<0.05, ***: P<0.001.
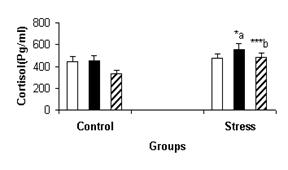
Figure-2: Comparison of the serum cortisol concentration in males (▒), and females
[follicular (c) and luteal (g) phases] in
the stress and control conditions. a: significant
difference in luteal phase under stress and control
conditions. b: significant difference for males in
control and stress conditions. *: P<0.05, ***: P<0.001.
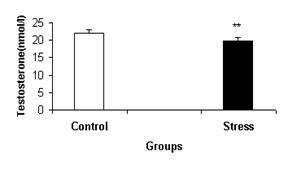
Figure-3: Comparison of serum
testosterone in males under stress (g), and control condition (c). **:Significant difference between
control and stress, P<0.01.
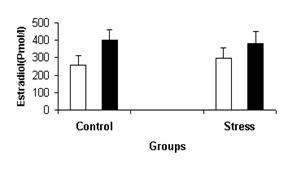
Figure-4: Mean ± SEM of serum estradiol of females’ follicular (c), and luteal (g) phases in control and stress situation.
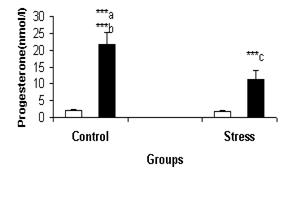
Figure-5: Serum progesterone
comparison in females’ follicular (c) and luteal (g) phases in control and stress conditions. a:
significant difference between stress and control situations in luteal phase. b: significant
difference between follicular and luteal phases in
control condition. c: significant difference between
follicular and luteal phases in stress condition.
***: P<0.001.
DISCUSSION
The results of this study showed
that: there were no differences in BMI of male and female groups at control and
stress conditions. Subsequent analysis
of sex diferences showed that males had higher systolic and diastolic blood
pressure and increase in heart rate in stress condition, compare to those of
females. Whereas, females had higher
respiratory frequency in stress condition compare to that’s of males. Moreover, there were no differences in SBP,
DBP and HR responses to stress condition, during the menstrual cycle. Our finding in cardiovascular responses is in
agreement with the studies of Matthews, that reported sex differences in SBP
and DBP responses during stress in middle age individuals6 and
Tersman that found increasing in SBP11, in this study, the
cardiovascular responses to physical and mental stresses in both menstrual
cycle phases has been examined in psychiatric female students. Zeller et al. have recently shown that during
medical licensing examination, DBP was significantly icreased but the SBP did
not change significantly.12
The present report is shown that, the sex differences also exsist in
young adult individuals, but is in oppose to the result of Bijlani.13
It is supposed that the differences between our finding and Bijlani are due to
the situation of experiments, because, the Bijlani experiments were done one
week before examination, but our experiment was on the day of examination.
Heart
rate increasing in this study is in agreement with the results of Stoney5
and Matthews6 that determined the cardiovascular responses to
physical and psychological stresses, if so Collins14 and Tersman11
that reported heart rate in females is more than males during stress condition
but is opposed to the report of Zeller et al.12 It has been shown
that subchronic physiological stress in human increased alpha2-adrenergic
receptor densithy, which is related to stress-induced anxiety.15
Examination stress cause a decrease in the parasympathetic influences on the
heart rate.16 Examination stress also changes the activity of
sympathetic and parasympatheticnervous system.17 Our finding in
respiratory frequency (RF) suggests that increasing in RF along with increasing
the activity of vagus nerve instead of accompanying with decrease of sympathic
activity, it is also possible that females may be more sensitive to stimulation
of vagus nerve than males. In the other
part of this study it was shown that cardiovascular responses are independent
from different phases of menstrual cysle both in control and stress
conditions. This result is consistent to
the Tersman study that has been reported there is no phase dependent difference
in SBP and DBP or heart rate under mental stress.11
Our results showed that
the plasma ACTH in male students is higher than females in both control and
stress conditions. Also the level of
cortisol in males increased under stress, whereas in females it was not
changed. These results are in agreement
with the results of other investigators.4,11,19 The results of this
study are also in areement with the finding of Martinek18 who
studied the effect of routine written examination on salivary cortisol and
Johansson that has shown the hormonal changes in male and female medical
students in response to examination stress.20 Furthermore Komesaroff
showed that only under stress condition the amount of cortisol in luteal phase
is greater than follicular phase, whereas in control condition there is no
significant difference, that is parallel with our finding,21 our
results is also simillar to Tersman finding that has measured the contisol
concentration in different phases.11
In other part of study
we measured the changes of sex hormones in response to examination stress. The
results showed that stress induced a significant decrease in male serum
testosterone compare to control, this result is consistent to previous study
that reported stress inhibits testosterone secretion in males.1,13
The possible mechanism of stress inhibitory effect on testosterone secretion is
due to the effect of stress on the hypothalamus–pituitary–gonad (HPG) axis,
because, gonadotropins secretion are correlated to the secretion of CRH, b-endorphins, ACTH, and
glucocorticoids. In females, serum progesterone response to stress reperesented
a significant decrease in luteal phase.
This suggests that the changes in female’s sex hormone are a result of
stress inhibitory effect on HPG axis in women. Variation in the concentration
of progestrone under stress could be the results of alteration in opioids
secretion, decrease in ACTH secretion under the stress is an indication of the
inhibitory role of estrogen due to decreased progestrone during stress that
affect ACTH secretion.21
Based on the above
mentioned results we could conclude that there are differences in physiological
responses to examination stress between males and females, so that males showed
a greater increase in systolic and diastolic blood pressure and also cortisol,
in response to stress. Therefore we
suggest that when the examination is stressful, different effects of stress in
male and female students should be considered, even in female students it is
supposed that stress affect in luteal phase differently from follicular phase.
REFERENCES
1.
Brann DW, Mahesh, VB. Role of corticostroids in female reproduction. FASEB J 1991; 5: 2691-8.
2.
Berne, RM, Levy, MN. Physiology. 4th Ed. London: Mosby Year
Book;1988..
3.
Miller NE. Learning, stress and Psychosomatic symptoms. Acta Neuobiol Exp 1978; 36: 141-56.
4.
Redei E, Lifang L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid
feedback inhibition of ACTH secretion in ovariectomized rat: Effect of chronic
estrogen and progesterone. Neuroendocrinology
1994;60:113-23.
5.
Stoney CM, Davis MC, Matthews KA. Sex differences in lipid, lipoprotein,
cardiovascular, and neuroendocrine responses to the acute stress. Psychophysiology 1988;25: 624-51.
6.
Matthews KA, Stoney CM. Influences of sex and age on cardiovascular
responses during stress. Psychosom Med
1988; 50: 46-56.
7.
Saab PG, Matthews KA, Stoney
CM, McDonald RH. Premenopausal and postmenopausal
women differ in their cardiovascular and neuroendocrine
responses to behavioral stressors. Psychophysiology
1989;26:270-80.
8.
Parker G, Cai Y, Tan S,
Dear K, Henderson AS, Poh GT, Kwee
GC. Examination stress in Singapore primary schoolchildren: how compliance by
subjects can impact on study results. Acta Psychiatr Scand 2003;108(3):239-43.
9.
Chiu A, Chon, SY, Kimball AB. The response of skin
disease to stress: changes in the severity of acne vulgaris
as affected by examination stress. Arch
Dermatol 2003;139(7):897-900.
10.
Droogleever Fortuyn HA, Van Broekhoven F, Span PN, Backstrom
T, Zitman FG, Verkes RJ.
Effects of PhD examination stress on allopregnanolone
and cortisol plasma levels and peripheral
benzodiazepine receptor density. Psychoneuroendocrinology 2004;29(10):1341-4.
11.
Tersman Z, Collins A, Eneroth
P. Cardiovascular responses to psychological and physiological stress during
the menstrual cycle. Psychosom Med 1991;53:
185-97.
12.
Zeller A, Handschin D, Gyr N, Martina B, Battergay E. Blood pressure and heart rate of students
undergoing a medical licensing examination. Blood Press 2004; 13(1): 20-4.
13.
Bijlani RL, Sud S, Gandhi BM,
Tandon BM. Relationship of examination stress to serum lipid profile. Indian J Physiology Pharmacology 1986;30:22-30.
14.
Collins A, Frankenhaeuser M.
Stress responses in male and female engineering students. J Human Stress 1978; 4:43-8.
15.
Maes M, Van Gastel A, Delmeire
L, Kenis G, Bosmans E, Song C. Platelet alpha2-adrenoceptor density in humans:
relationships to stress-induced anxiety, psychathenic constitution, gender,and
stress-induced changes in the inflammatory response system. Psychol Med 2002;32(5):919-28.
16.
Makarenko MV, Lyzohub VS,
Iukhymenko LI. Heart rhythm in students with different individual and typological
characteristics of the higher nervous activity during examination stress. Fiziol Zh 2003; 49(1): 28-33.
17.
Faustov AS, Shcherbatykh IuV.
Changes in the functional state of the nervous system of students during
studies. Gig sanit 2000; 6:
33-35.
18.
Martinek L,
Oberascher-Holzinger K, Weishuhn S, Klimesch W, Kerschbaum HH. Anticipated
academic examinations induce distinct cortisol responses in adolescent pupils. Neuro Endocrinol 2003;24(6):449-53.
19.
Armario A, Marti O, Molina T, de Pablo J, Valdes M. Acute stress
markers in humans: response of plasma glucose, cortisol
and prolactin to two examinations differing in the
anxiety they provoke. Psychoneuroendocrinology 1996; 21(1): 17-24.
20.
Johansson GG, Laakso M, Peder
M, Karonen SL. Endocrine patterns before and after examination stress in males
and females. Act Nerv Super (Praha) 1989;31(20):
81-8.
21.
Komesaroff PA, Esler M, Clark
IJ, Fullerton MJ, Funder JW. Effect of estrogen and estrus cycle on
glucocorticoid and catecholamine response to stress in sheep. Am J Physiology (End Met) 1998; 275:
E671-8.
_____________________________________________________________________________________________
Address For Correspondence:
Dr. Mohammad Khaksari, Dept. of Physiology, Faculty of Medicine,
Kerman, Iran.Fax: ++98 341 3221671
Email: Khaksar38@yahoo.co.uk




